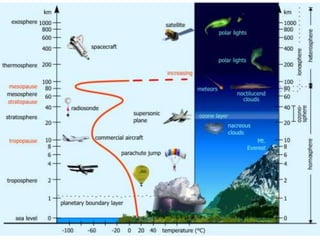
The Earth's atmosphere is a protective layer of gases that becomes thinner with altitude and is composed mainly of nitrogen and oxygen. It consists of several layers including the troposphere, stratosphere, mesosphere, thermosphere, and exosphere, each with distinct characteristics and structures such as the ozone layer and various types of clouds. Additionally, the document discusses air pollutants, their sources, and their impact on the environment and health.