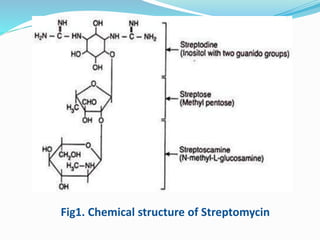
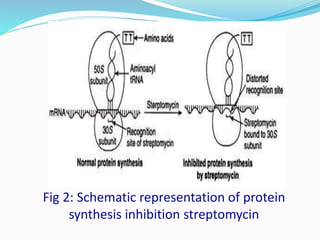
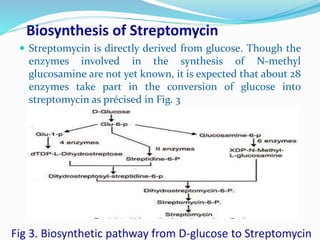
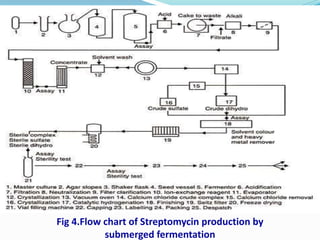
Streptomycin is an antibiotic discovered in 1944 that is produced through fermentation of Streptomyces griseus bacteria. It is used to treat infections caused by gram-positive and gram-negative bacteria as well as tuberculosis. Production occurs over 3 phases, beginning with rapid bacterial growth and ending with cell lysis and harvest. Streptomycin is recovered through adsorption onto activated carbon or ion exchange resins before precipitation and purification. It functions by binding to the bacterial ribosome and inhibiting protein synthesis.