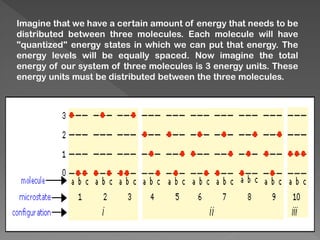
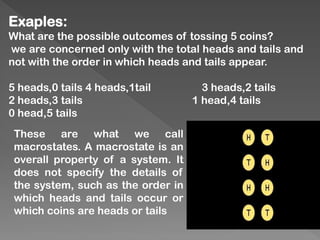
The document discusses microstates and macrostates in thermodynamics, explaining how microstates contribute to entropy and the likelihood of systems transitioning to higher entropy states. It also introduces Stirling's approximation, a mathematical formula for estimating factorials, detailing its derivation and significance. The connection between probability, disorder, and energy distribution is emphasized throughout the explanation.





![The equation can also be derived using the
integral definition of the factorial,
ln 𝑁! = 1
𝑁
1 ln 𝑛 𝑑𝑥
As we know the role of
Integration by parts
∫u v dx = u∫v dx −∫u' (∫v dx) dx
So, the above equation become
ln 𝑁! = [n ln n] 𝑁
1
-1
𝑁
𝑥.
1
𝑥
𝑑𝑥](https://image.slidesharecdn.com/stirlingsapproximationmicrostateandconfiguration-200530114902/85/Stirling-s-approximation-amp-microstate-and-configuration-8-320.jpg)
![ln 𝑁 = N ln N – 1 ln 1- 1
𝑁
1 𝑑𝑥
ln𝑁 = N ln N – 0 - 1
𝑁
1 𝑑𝑥
= N ln N – [x] 𝑁
1
=N ln N – [N-1]
= N ln N –N + 1
We Can neglect 1 because there is large quantity
present so the is no effect on the eq.
Now solve the values of limits of integration](https://image.slidesharecdn.com/stirlingsapproximationmicrostateandconfiguration-200530114902/85/Stirling-s-approximation-amp-microstate-and-configuration-9-320.jpg)
