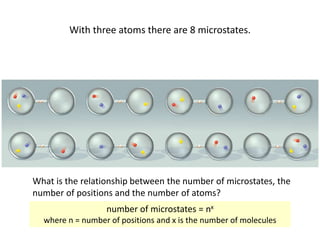
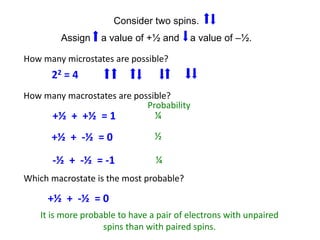
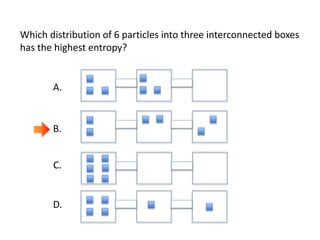
Entropy is a measure of the dispersal of energy in a system as a function of temperature. It is related to the number of microscopic configurations or "microstates" that are consistent with the macroscopic properties of the system. The more possible microstates there are, the higher the entropy. According to the second law of thermodynamics, isolated systems spontaneously evolve towards configurations with higher probability and maximum entropy.