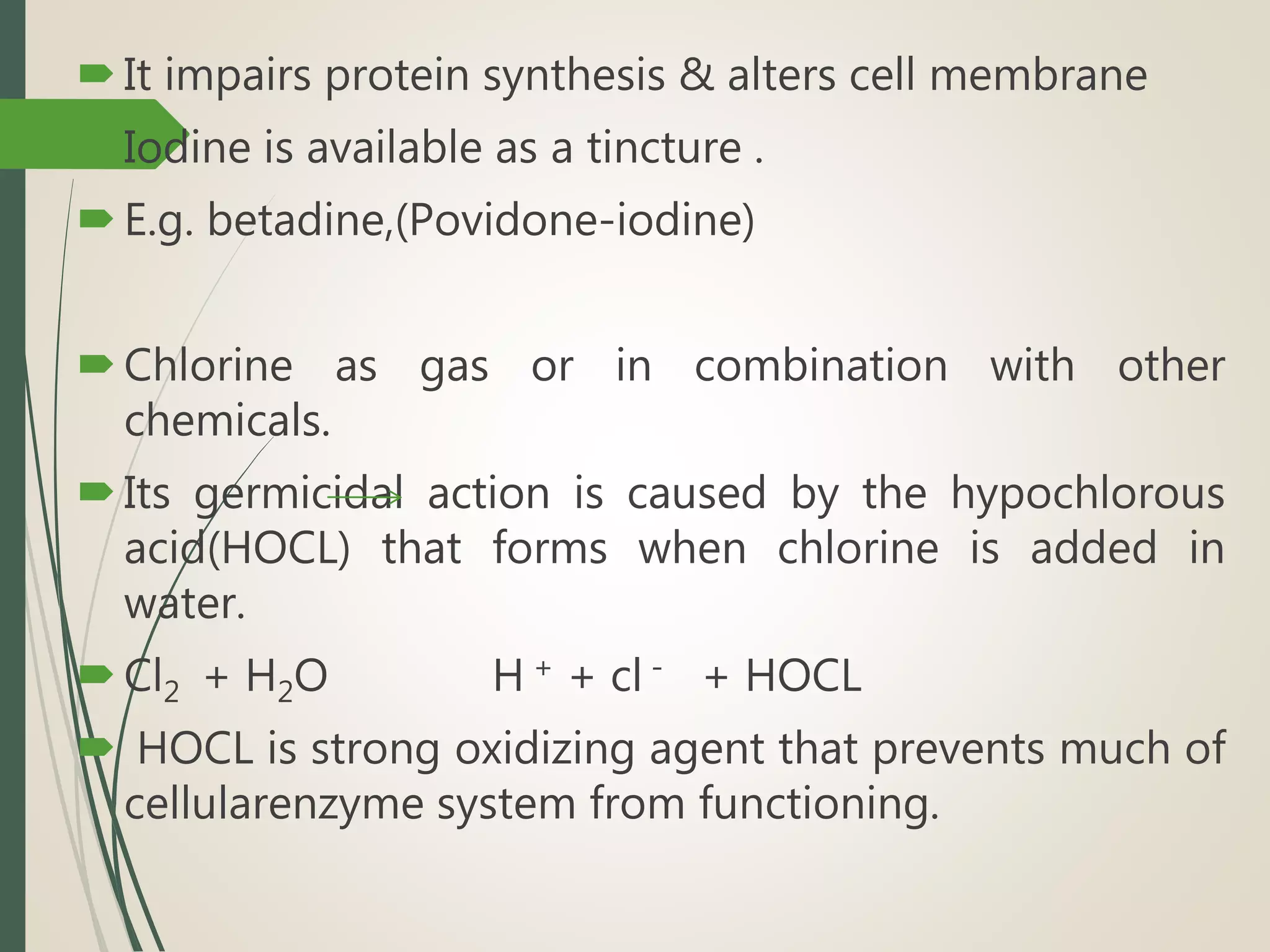
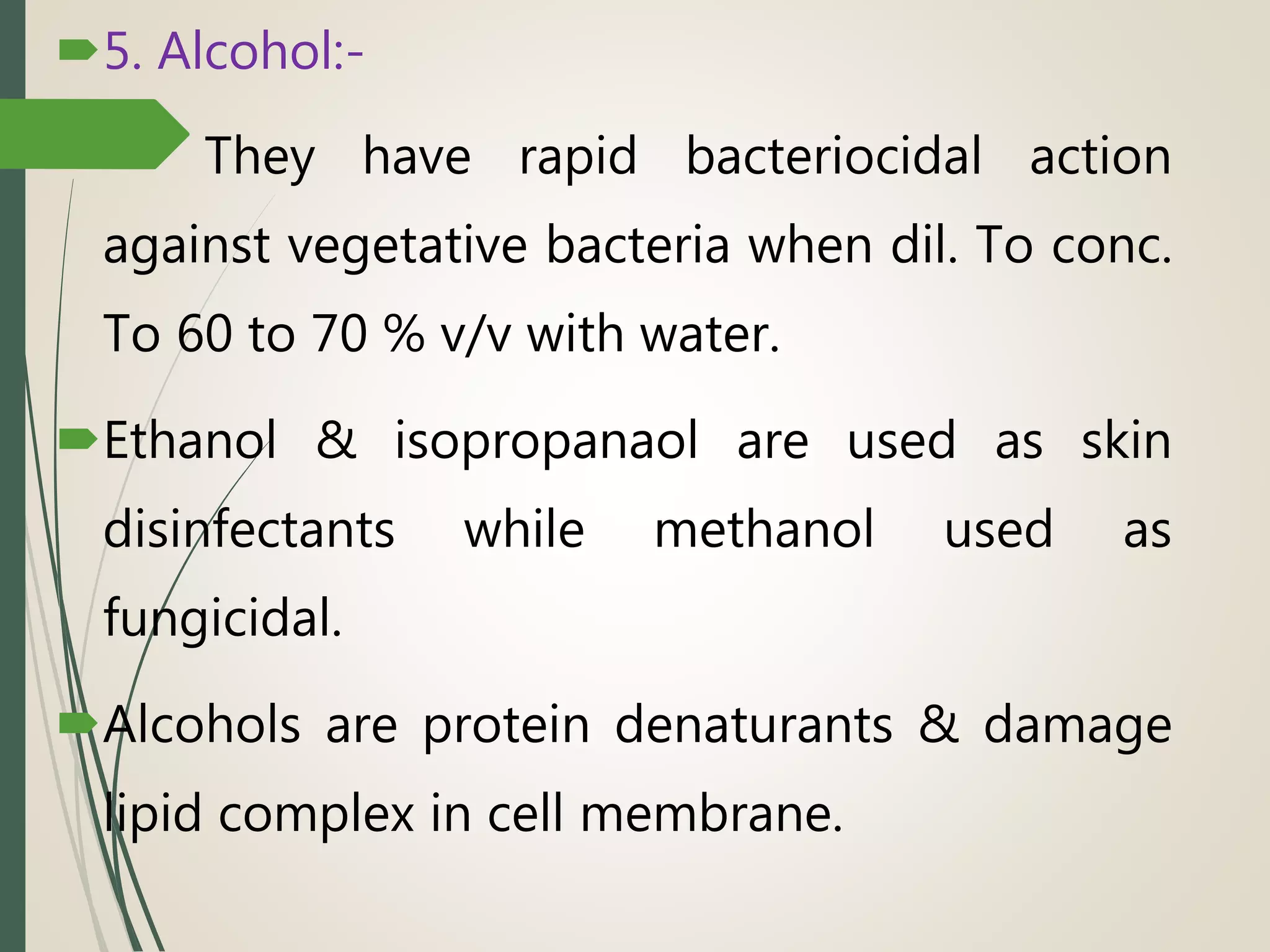
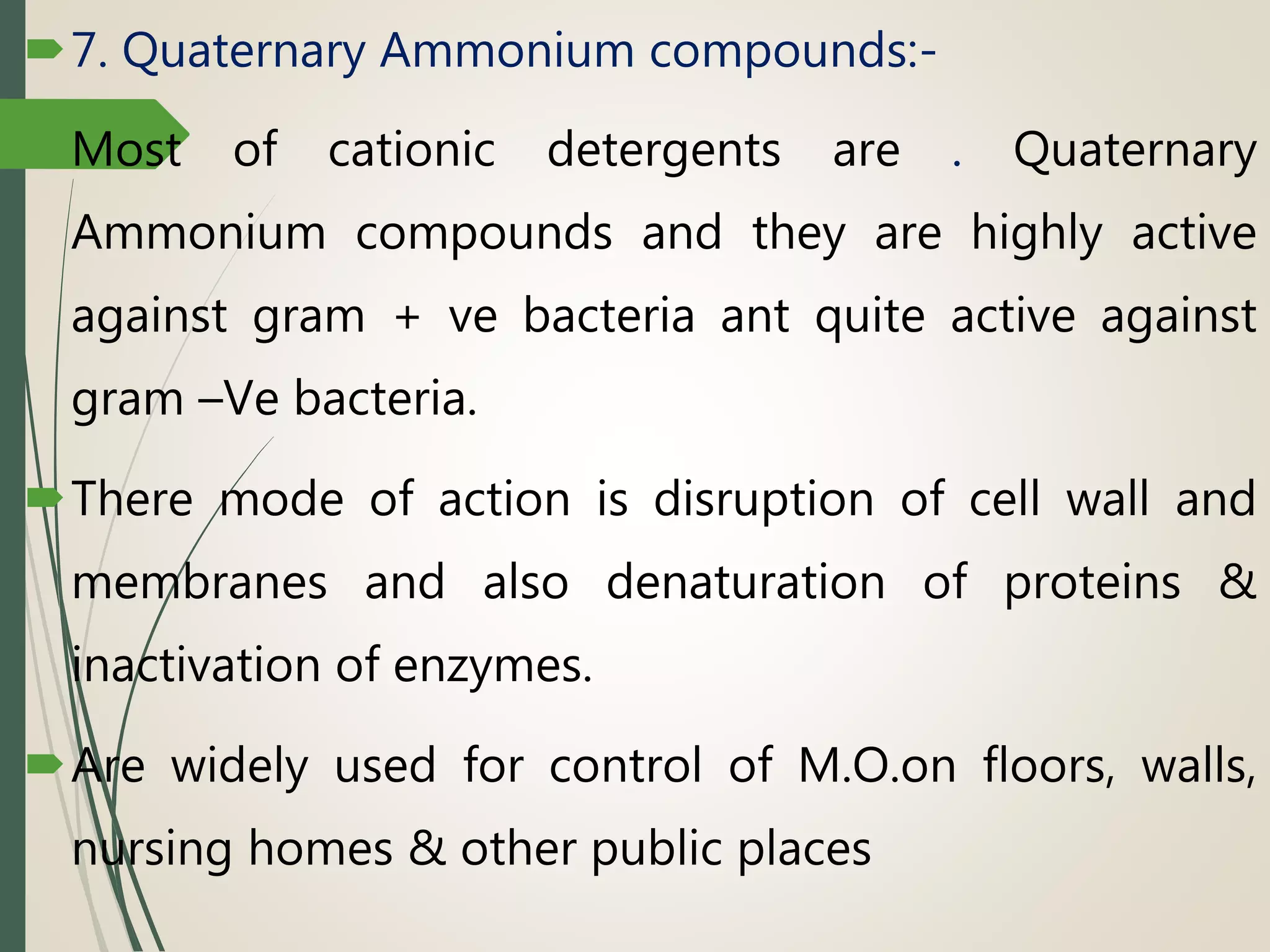
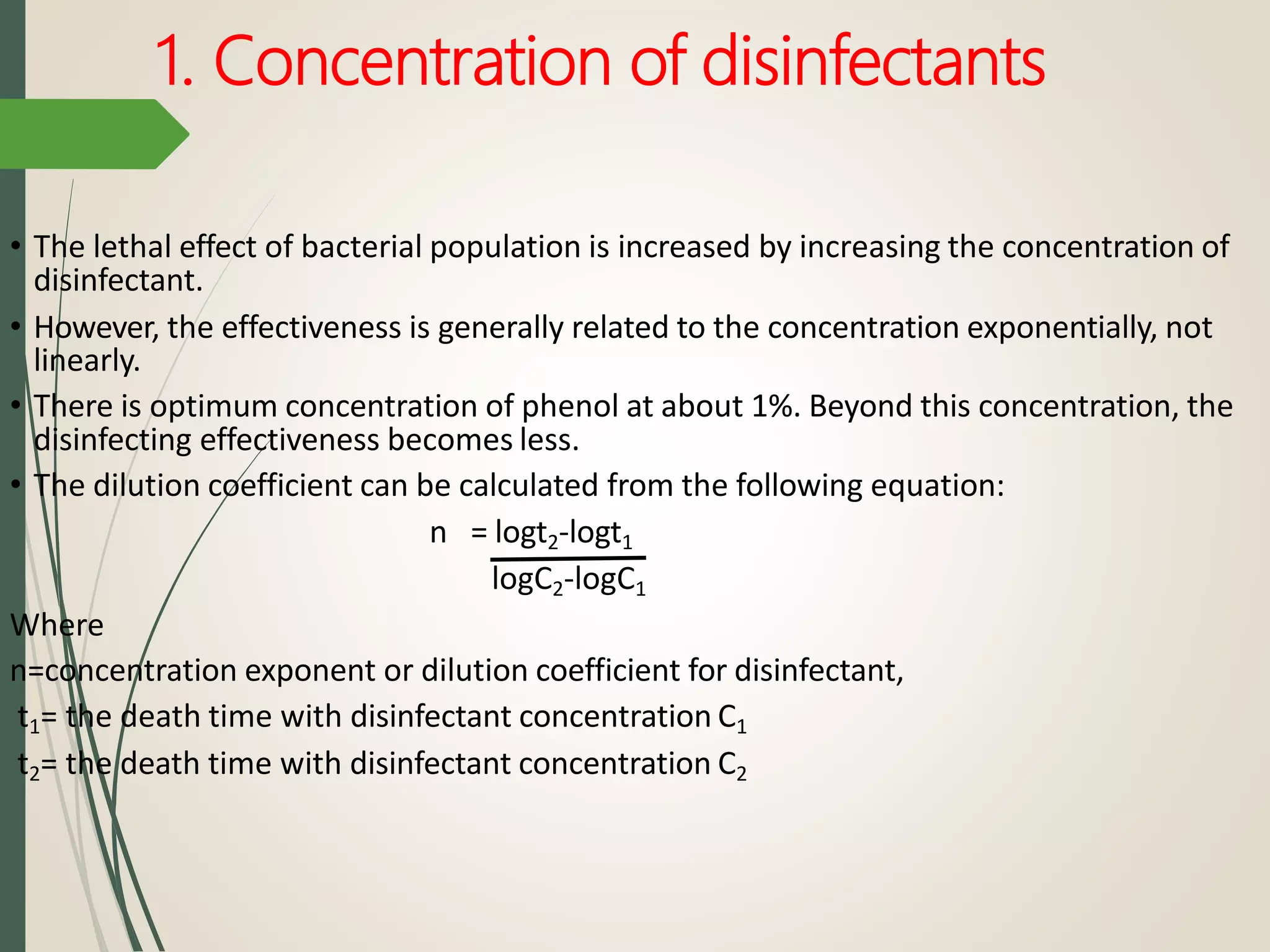
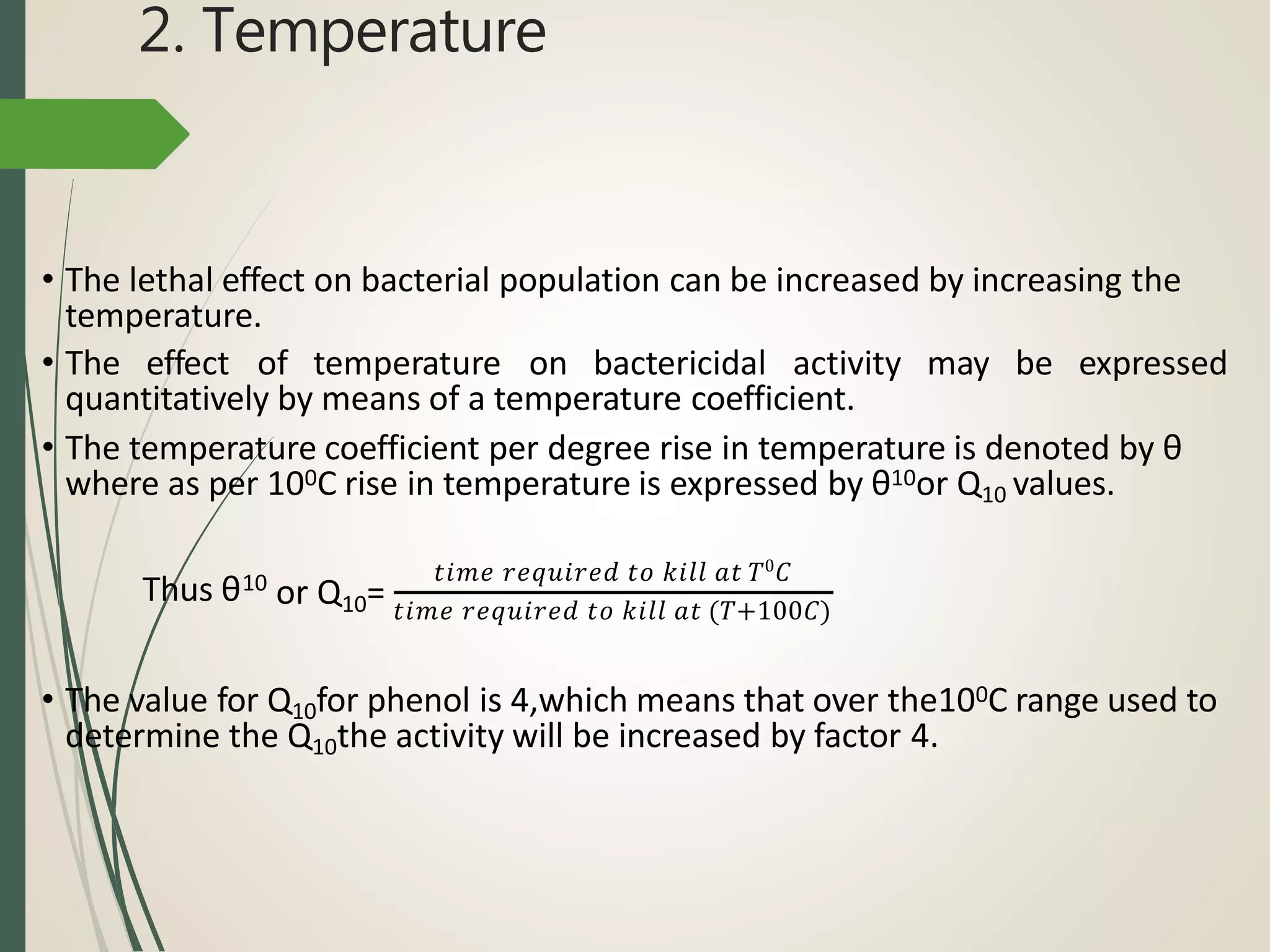
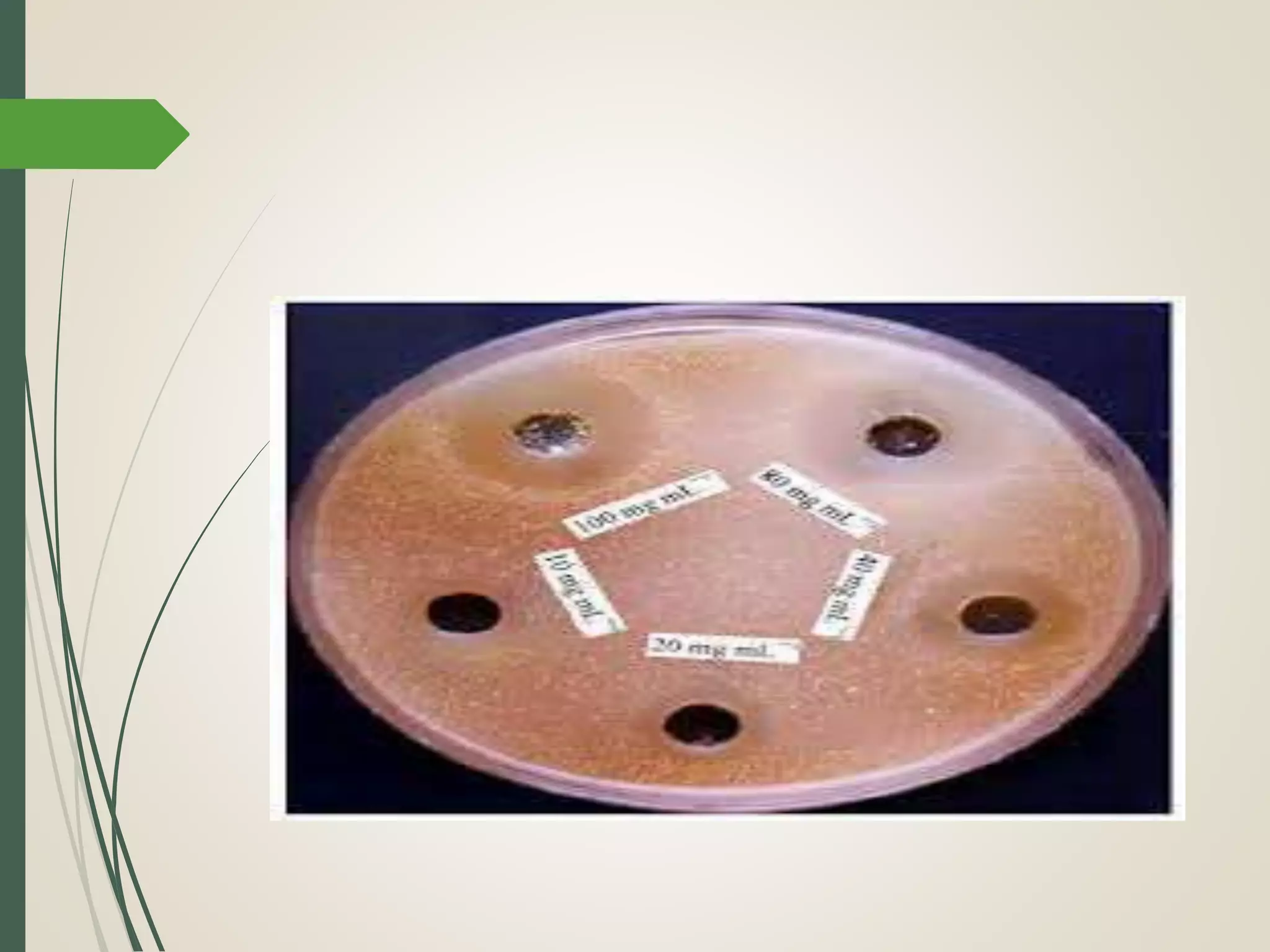
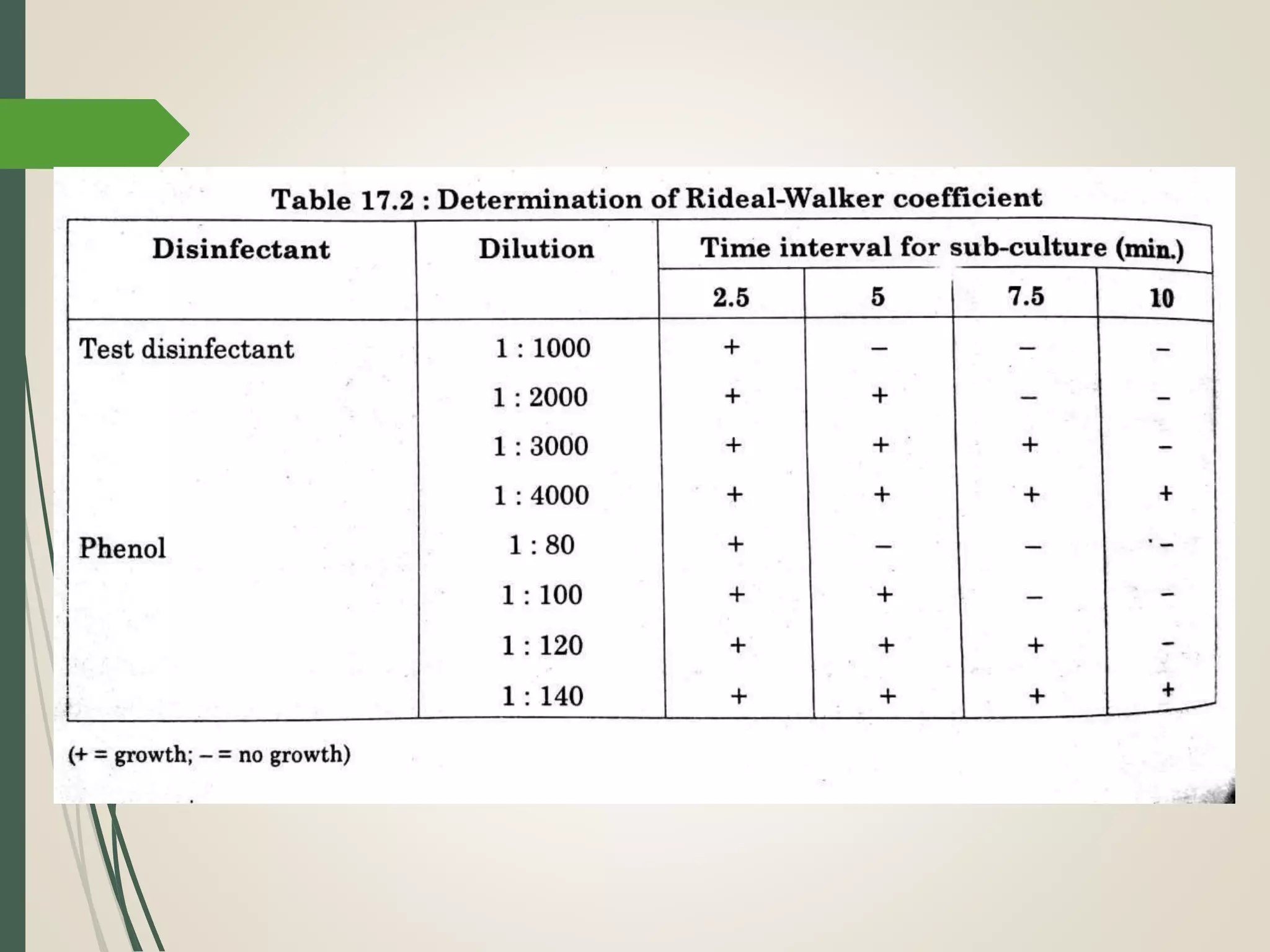
The document discusses various disinfectants, their chemical classifications, and mechanisms of action against microorganisms. It highlights the importance of concentration, temperature, contact time, and environmental factors on disinfectant efficacy. Evaluation techniques for antimicrobial agents are also covered, including tube dilution and phenol coefficient tests.