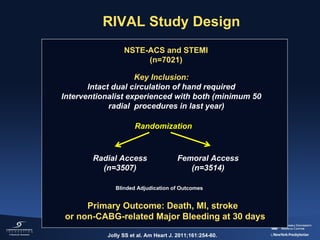
1) The document summarizes evidence from multiple studies comparing radial versus femoral access for ST-elevation myocardial infarction (STEMI) patients undergoing primary percutaneous coronary intervention (PCI).
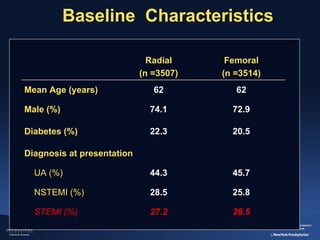
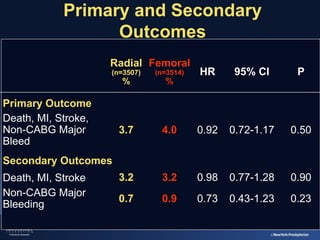
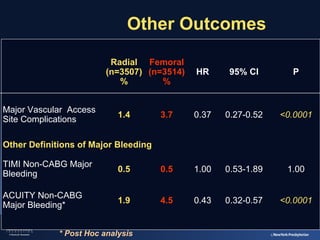
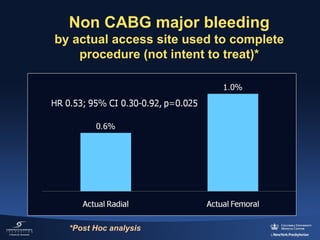
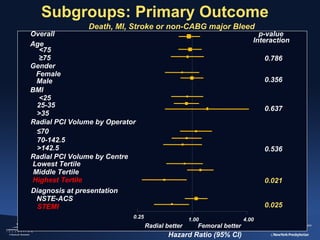
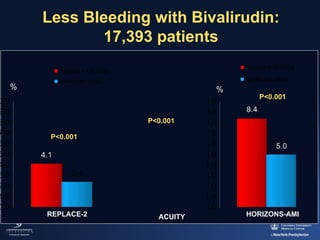
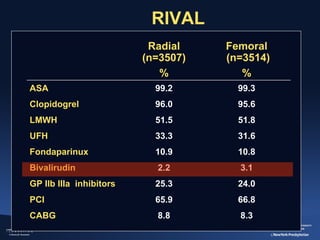
2) The RIVAL trial found no difference in major clinical outcomes between radial and femoral access but found significantly lower rates of major bleeding and vascular complications with radial access. Subgroup analysis found radial access was associated with lower rates of death and bleeding in STEMI patients.
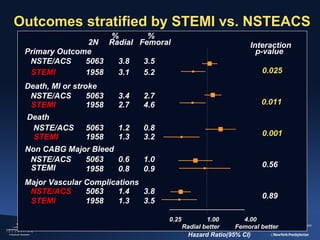
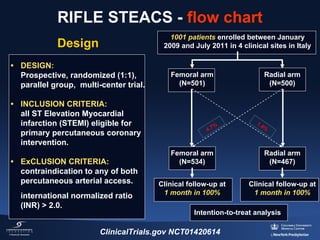
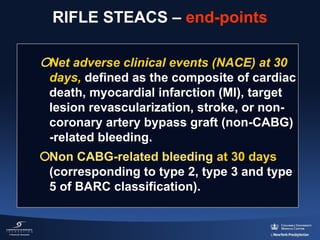
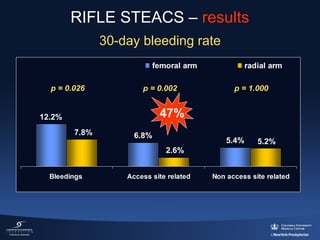
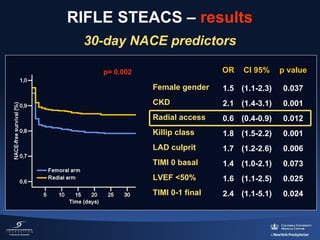
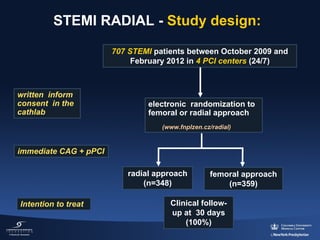
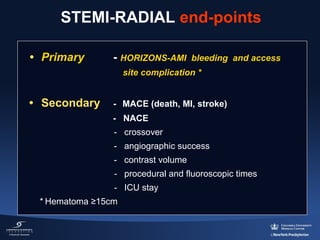
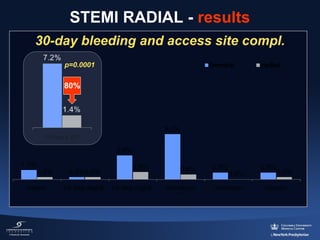
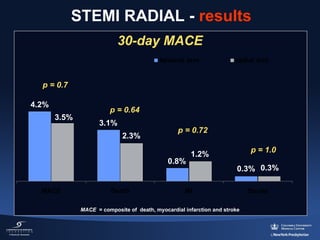
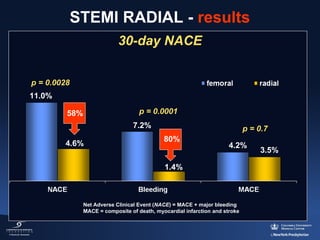
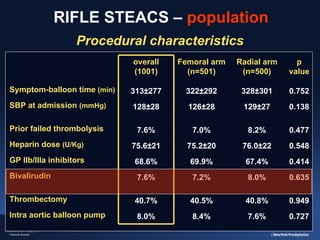
3) The RIFLE STEACS and STEMI-RADIAL trials both found significantly lower rates of bleeding, with RIFLE STEACS also finding lower mortality, with radial versus femoral access in STEMI patients undergoing primary PCI.









![HORIZONS-AMI: 30-Day Mortality
Death (%)
Heparin + GPIIb/IIIa inhibitor (n=1802)
Bivalirudin monotherapy (n=1800)
3.1%
2.1%
HR [95%CI] =
0.66 [0.44, 1.00]
P=0.048
Number at risk
Bivalirudin
Heparin + GPIIb/IIIa
Time in Days
1800
1802
1758
1764
1751
1748
1746
1736
1742
1728
Stone, G et al. NEJM 2008;358:2218-2230
1729
1707
1666
1630](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-29-320.jpg)
![Kaplan-Meier Curves of 1-Year Cumulative
Major Bleeding (non-CABG Related)
Major Bleed (%)
15
Radial
Femoral
10
8.1%
5
3.5%
HR: 0.42 [95% CI: 0.20, 0.89]
p= 0.019
0
0
1
2
3
4
5
6
7
8
9
10
11
12
Time in Months
Number at risk
Radial
Femoral
200
3134
188
2785
188
2753
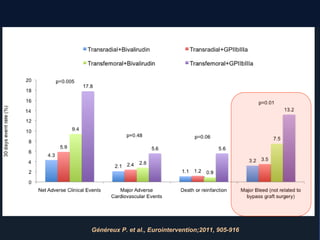
Généreux P. et al., Eurointervention;2011, 905-916
187
2716](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-33-320.jpg)
![Kaplan-Meier Curves of 1-Year Cumulative
Death/MI
Death/MI (%)
15
Radial
Femoral
10
7.8%
5
4.0%
HR: 0.51 [95% CI: 0.25, 1.02]
p= 0.052
0
0
1
2
3
4
5
6
7
8
9
10
11
12
Time in Months
Number at risk
Radial
Femoral
200
3134
193
2920
192
2864
Généreux P. et al., Eurointervention;2011, 905-916
191
2812](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-34-320.jpg)
![Kaplan-Meier Curves of 1-Year
Cumulative MACE
MACE (%)
20
Radial
Femoral
15
12.4%
10
6.0%
5
HR: 0.47 [95% CI: 0.26, 0.83]
p= 0.008
0
0
Number at risk
Radial
Femoral
200
3134
1
2
3
4
191
2844
5
6
7
Time in Months
8
9
10
11
190
2753
Généreux P. et al., Eurointervention;2011, 905-916
12
187
2673](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-35-320.jpg)
![Kaplan-Meier Curves of 1-Year
Cumulative NACE
NACE (%)
20
Radial
Femoral
17.8%
15
10
8.5%
5
HR: 0.45 [95% CI: 0.28, 0.74]
p= <.001
0
0
Number at risk
Radial
Femoral
200
3134
1
2
3
4
186
2669
5
6
7
Time in Months
8
9
10
185
2587
Généreux P. et al., Eurointervention;2011, 905-916
11
12
182
2511](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-36-320.jpg)
![Multivariable Analysis
30 days and 1-year
30 days analysis
Hazard Ratio
p value
NACE
Radial (vs. Femoral)
0.31 [ 0.15, 0.66]
<0.01
MACE
Radial (vs. Femoral)
0.33 [ 0.10, 1.04]
0.058
Major Bleeding
(non-CABG related)
Radial (vs. Femoral)
0.40 [ 0.18, 0.90]
0.02
1-year analysis
Hazard Ratio
p value
NACE
Radial (vs. Femoral)
0.40 [ 0.22, 0.74]
<0.01
MACE
Radial (vs. Femoral)
0.46 [ 0.23, 0.93]
0.03
Major Bleeding
(non-CABG related)
Radial (vs. Femoral)
0.27 [ 0.07, 1.09]
0.06
The following potential covariates were included in the model : age, sex, race, BMI, Killip class, baseline anemia (defined as baseline hematocrit below 39% for men and 36% for
women), platelet counts, creatinine clearance <60ml/min, white blood cell count, left ventricular ejection fraction, hypertension, hyperlipidemia, smoking, diabetes, insulino-dependent
diabetes, previous MI, previous CABG, previous CAD (coronary artery disease), angina, heart failure, peripheral vascular disease, clopidogrel loading dose, pre-randomization heparin,
baseline and discharge aspirin, baseline and discharge thienopyridines, randomization to bivalirudin vs. UFH+GP, symptom to first balloon time, LAD disease and access site (Radial
vs. Femoral).](https://image.slidesharecdn.com/stemiviaradial-possibleandpreferred-131113083518-phpapp01/85/Genereux-P-STEMI-via-radial-37-320.jpg)