This document discusses chemical kinetics and reaction rates. It begins by defining key terms like reaction rate, rate laws, half-life, and the Arrhenius equation. It then discusses methods for determining the order of reactions, such as zero-order, first-order, and second-order reactions. Specific equations are provided for calculating rate constants and half-lives for each reaction order. The document emphasizes the importance of understanding reaction kinetics for applications like predicting drug stability and dissolution.





![APPARENT ZERO ORDER - SUSPENSIONS
It may be first order but
behaves like a zero order,
depending on the
experimental conditions.
Degradation is possible only
when drug is available in
solution form.
As the drug in solutions starts
to degrade suspended
particles act as reservoir &
continuously release drug in
solution. Thus con. Remains
constant throughout the
process.
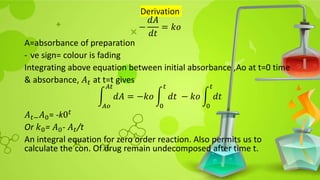
In case of no reservoir; −
𝑑 𝐴
𝑑𝑡
=
𝑘1[𝐴]
]](https://image.slidesharecdn.com/chemicalkineticspp-230424134535-f34d57bb/85/Chemical-kinetics-pp-pptx-11-320.jpg)
![FIRST ORDER REACTION
• −
𝑑𝑐
𝑑𝑡
∝ 𝑐 or −
𝑑𝑐
𝑑𝑡
= 𝑘1𝑐
Derivation
A Products
According to definition of 1st order,
−
𝑑𝑐
𝑑𝑡
= 𝑘1𝑐
𝑐0
𝑐1
𝑑𝑐
𝑐
= −𝑘1
𝑜
𝑡
𝑑𝑡
[𝐼𝑛 𝑐]𝑐𝑜
𝑐𝑡
=-k1[𝑡]0
𝑡](https://image.slidesharecdn.com/chemicalkineticspp-230424134535-f34d57bb/85/Chemical-kinetics-pp-pptx-12-320.jpg)

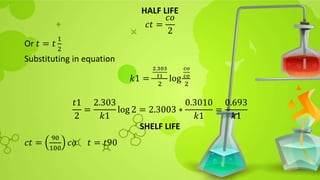
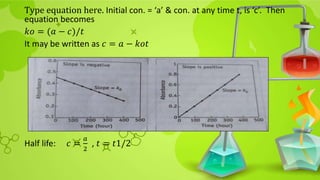
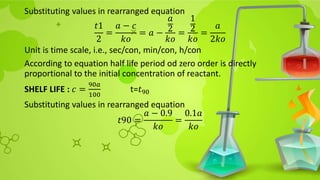
![Substituting in rearranged equation
𝑡90 =
2.303
𝑘1
log
𝑐𝑜
0.9𝑐0
𝑡90 =
2.303
𝑘1
log
10
9
= 2.303 ∗
0.04575
𝑘1
=
0.105
𝑘1
Pseudo first Order Reaction
−
𝑑𝑐
𝑑𝑡
= 𝑘2[𝐴][𝐵]
A& B= reactants, K2= 2nd order rate constant
Reaction conditions are maintained such that B present in excess
amount compared to A. That’s why equation changes to
−
𝑑𝑐
𝑑𝑡
= 𝑘2 𝐴 𝑐𝑜𝑛𝑠𝑡𝑎𝑛𝑡 = 𝑘1[𝐴]](https://image.slidesharecdn.com/chemicalkineticspp-230424134535-f34d57bb/85/Chemical-kinetics-pp-pptx-15-320.jpg)
![SECOND ORDER REACTION
A+B=Products
Rate equation can be written as:
−
𝑑𝐴
𝑑𝑡
= −
𝑑𝐵
𝑑𝑡
= 𝑘2[𝐴]1[𝐵]1
[A] & [B]= concentration of A & B respectively
K2= Specific rate constant for 2nd order.
i.e. Rate of reaction is 1st order W.R.T. A & again 1st order W.R.T B.
Therefore, m+n=2
For identification/evidence purpose; Plot graph of log con. Vs. time.
Straight line indicating 1st order w.r.t A & again 1st order w.r.t B.](https://image.slidesharecdn.com/chemicalkineticspp-230424134535-f34d57bb/85/Chemical-kinetics-pp-pptx-16-320.jpg)
![DERIVATION
−
𝑑𝐴
𝑑𝑡
= −
𝑑𝐵
𝑑𝑡
= 𝑘2[𝐴][𝐵]
Let ‘a’ & ‘b’ be the initial con. Of A&B, and ‘x’ be the con. Of each
species reacting in time t. On substituting these gives,
𝑑𝑥
𝑑𝑡
= 𝑘2 𝑎 − 𝑥 (𝑏 − 𝑥)
By considering case in which a=b i.e. (both having same con.) then the
equation will be
𝑑𝑥
𝑑𝑡
= 𝑘2(𝑎 − 𝑥)2
Integrating above equation on employing the conditions x=0 at t=0 &
x=x at t=t, gives
0
𝑥
𝑑𝑥/(𝑎 − 𝑥)2 = 𝑘2
0
𝑡
𝑑𝑡](https://image.slidesharecdn.com/chemicalkineticspp-230424134535-f34d57bb/85/Chemical-kinetics-pp-pptx-17-320.jpg)