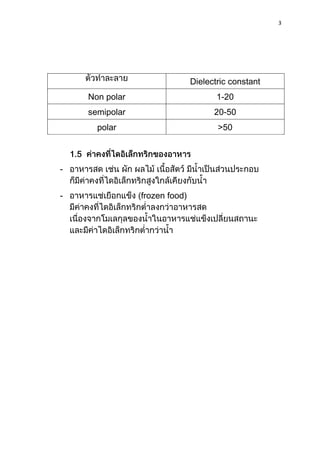
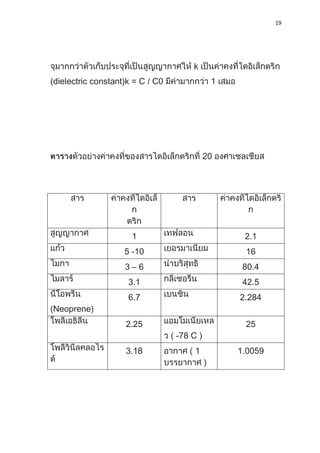
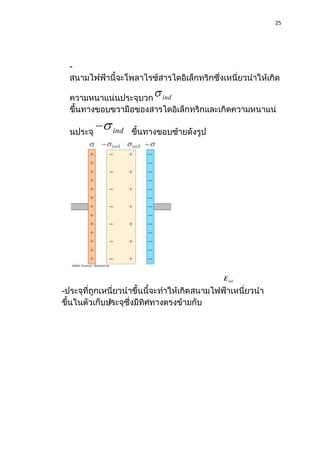
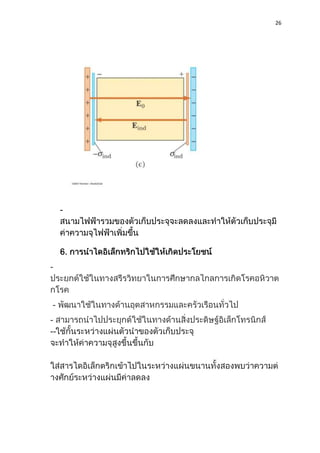
1) Dielectric materials have a dielectric constant (ε) which describes their ability to be polarized by an external electric field. Materials with higher dielectric constants store more electrical energy.
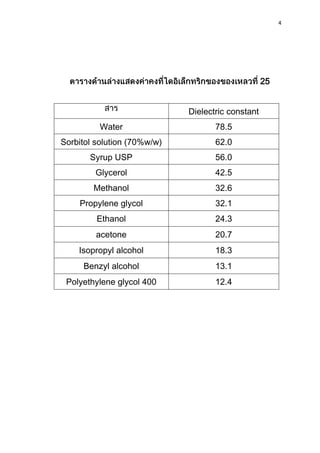
2) The dielectric constant of water is 78.5, much higher than most other materials like glycerol at 42.5 or ethanol at 24.3. This is why water is commonly used as a dielectric in capacitors.
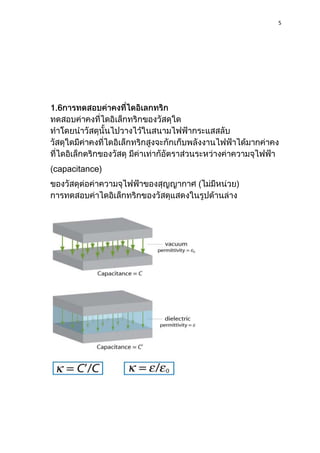
3) The dielectric constant determines the capacitance of a capacitor. Capacitance increases as the dielectric constant increases for a given capacitor geometry.