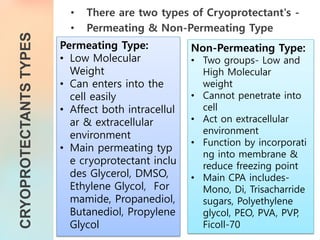
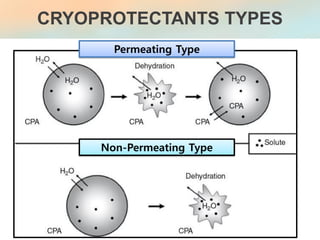
The document discusses the process and implications of sperm freezing (cryopreservation), including its historical context, techniques, and the effects on sperm quality. It outlines the procedures for sperm collection, storage, and thawing while highlighting the advantages and challenges associated with cryopreservation, including potential damage to sperm function and viability. Overall, sperm cryopreservation is a widely used technique in fertility clinics, with continued advancements improving outcomes for future fertility.