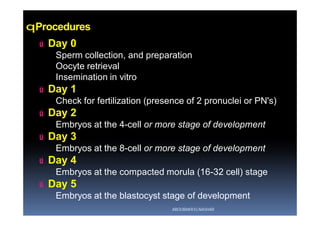
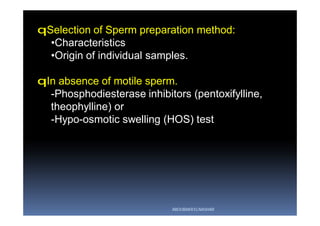
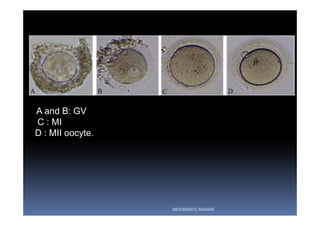
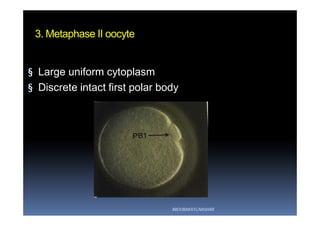
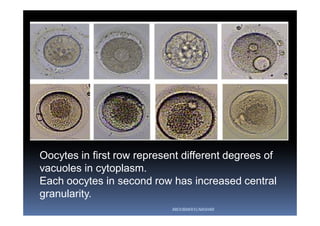
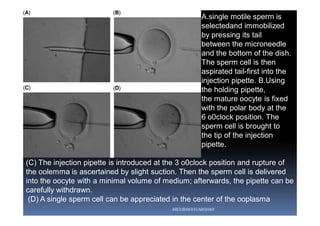
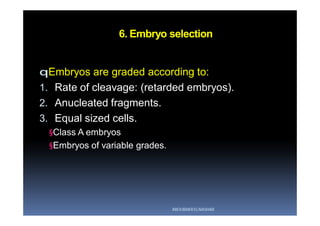
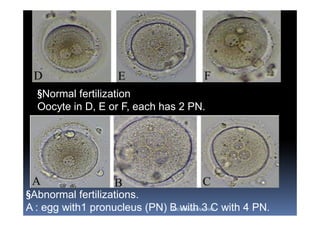
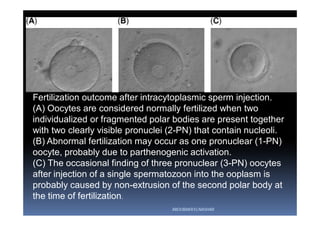
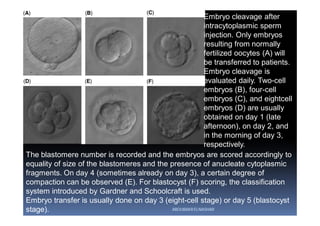
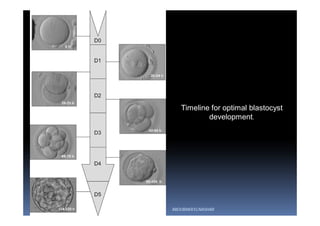
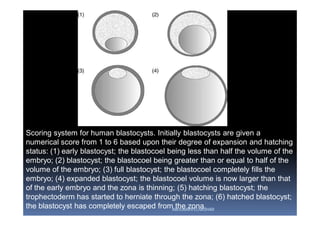
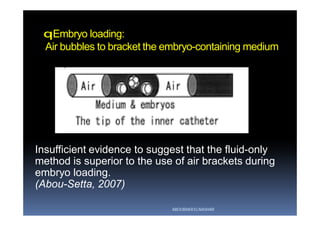
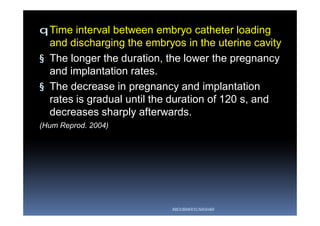
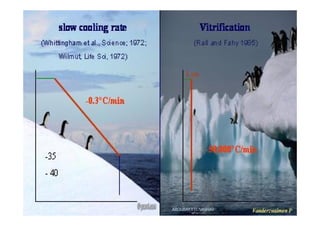
The document outlines the key procedures involved in ICSI lab work for a gynecologist, as presented by Aboubakr Elnashar from Benha University in Egypt. It discusses 8 main procedures: 1) semen preparation, 2) oocyte identification, 3) oocyte denudation, 4) oocyte assessment, 5) oocyte injection, 6) embryo selection, 7) embryo transfer, and 8) cryopreservation. For each procedure, it provides details on the aim, methods, and considerations. The document serves as a reference for best practices in ICSI lab work to optimize outcomes.