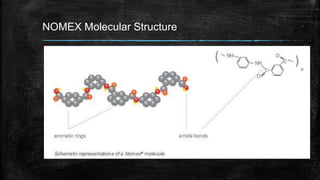
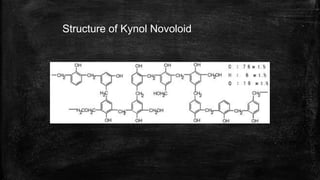
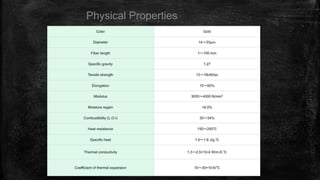
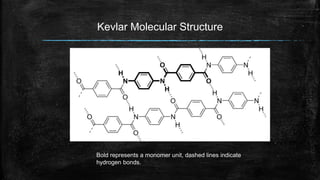
The document provides detailed information about various special use fibers, including Nomex, Novoloid, Saran, PVC, Spandex, and Kevlar, highlighting their production methods and properties. It emphasizes the flame-retardant qualities of Nomex, the chemical resistance of Saran, and the elasticity of Spandex, alongside industrial and military applications of these materials. Additionally, it discusses the advantages and disadvantages of each fiber type, along with their uses in safety products and textiles.