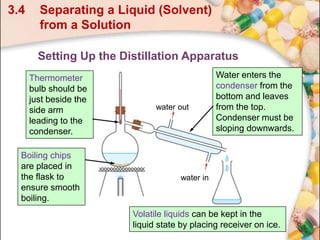
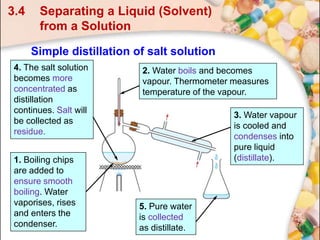
This document discusses methods for separating and purifying mixtures. Section 3.4 focuses on separating a liquid solvent from an aqueous solution using simple distillation. Simple distillation involves boiling the solution so that the solvent, having a lower boiling point than the solute, vaporizes and condenses as a pure liquid distillate. For example, water can be separated from a sugar solution or potassium bromide solution by heating the mixture to vaporize the water, which is then cooled and collected as pure distillate, leaving behind a more concentrated solution of the solute.