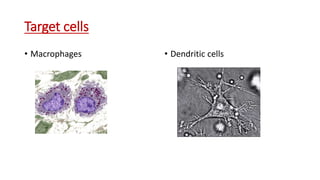
Simian Hemorrhagic Fever is a viral hemorrhagic fever that causes lethal disease in Asian macaque monkeys resembling Ebola or Lassa fever in humans. It is characterized by the release of cytokines from infected macrophages and dendritic cells in the liver and spleen, inducing coagulation issues. Two outbreaks occurred in 1964 among captive macaques. The virus is an arterivirus that infects macrophages and dendritic cells. It is highly contagious and fatal, causing fever, edema, bleeding, and death within 7-13 days. There is no specific treatment but symptoms can be treated.