This document summarizes information about silver, including its discovery, extraction and purification processes, properties, applications, and common alloys. Some key points:
- Silver was discovered as early as 3000 BC and is found in nature as a pure metal or mixed with other metals like gold, copper, and lead.
- Extraction involves crushing ore, smelting, and separating silver through processes like treating sludge with nitric acid.
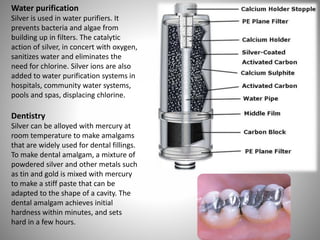
- Silver has many industrial and commercial uses like electrical contacts, jewelry, coins, photography, and water purification due to its conductive and antimicrobial properties.
- Common silver alloys include sterling silver (92.5% silver), Britannia silver (95.83% silver),