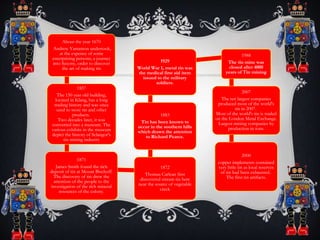
Tin is a post-transition metal that is obtained chiefly from the mineral cassiterite. It has many important uses including coating other metals to prevent corrosion in tin cans and in alloys like solder and bronze. Tin extraction dates back to the Bronze Age and played a key role in the development of civilization through its use in bronze tools and weapons. It has a silvery appearance and low melting point of 232°C.

![Group 14
and
Period 5 in Periodic Table
Relative Atomic Mass
118.710
Key isotopes
120Sn
Electron configuration
[Kr] 4d105s25p2
Atomic number
50](https://image.slidesharecdn.com/elementoftin-170903122632/85/Element-of-tin-2-320.jpg)


![ETYMOLOGY
The word tin is shared among Germanic languages and can be traced back
to reconstructed Proto-Germanic *tin-
om; cognates include German Zinn, Swedish tenn and Dutch tin. It is not found in other
branches of Indo-European, except by borrowing from Germanic (e.g. Irish tinne from
English).
The Latin name stannum originally meant an alloy of silver and lead, and came to
mean 'tin' in the 4th century BCE the earlier Latin word for it was plumbum candidum, or
"white lead". Stannum apparently came from an earlier stāgnum (meaning the same
substance),] the origin of the Romance and Celtic terms for tin. The origin
of stannum/stāgnum is unknown; it might be pre-Indo-European.](https://image.slidesharecdn.com/elementoftin-170903122632/85/Element-of-tin-6-320.jpg)









![MINE RESERVES
World tin mine reserves (tonnes, 2011)[40]
Country Reserves
China 1,500,000
Malaysia 250,000
Peru 310,000
Indonesia 800,000
Brazil 590,000
Bolivia 400,000
Russia 350,000
Australia 180,000
Thailand 170,000
Other 180,000
Total 4,800,000](https://image.slidesharecdn.com/elementoftin-170903122632/85/Element-of-tin-16-320.jpg)
