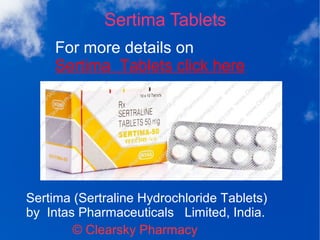
Sertima (sertraline hydrochloride tablets) is an SSRI used for treating depression, OCD, panic disorder, social phobia, premenstrual dysphoric disorder, and PTSD. The tablets are available in 50 mg and 100 mg doses and are contraindicated for patients with hypersensitivity to the drug or who are taking MAO inhibitors. Side effects may include nausea, diarrhea, and tremor, with withdrawal symptoms occurring upon sudden discontinuation.