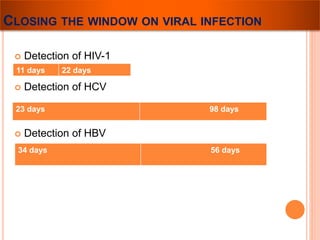
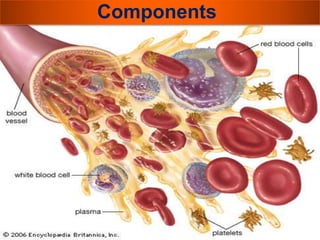
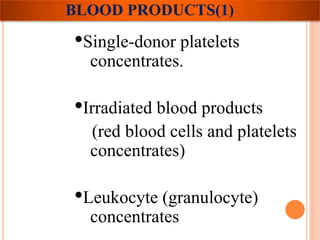
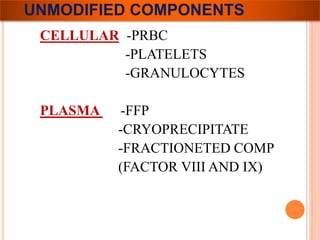
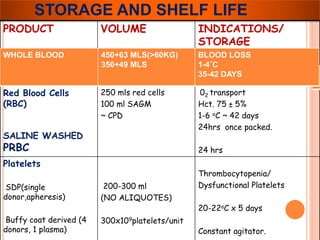
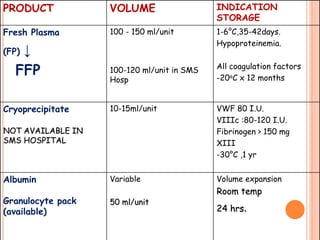
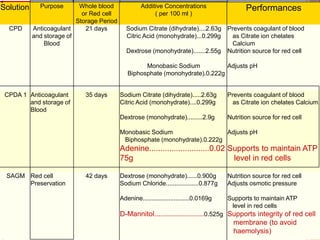
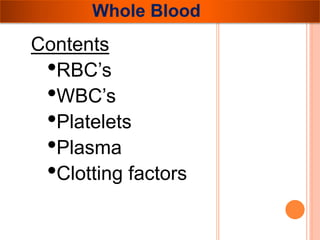
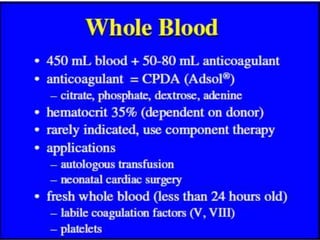
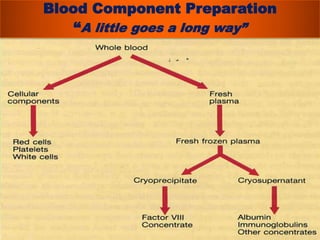
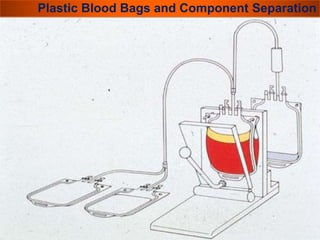
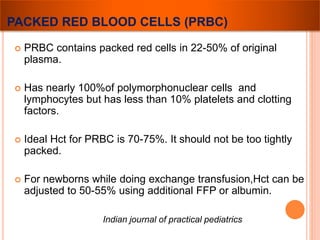
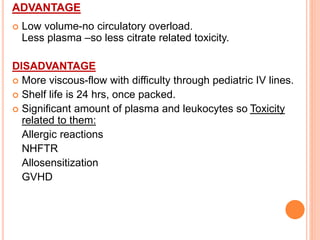
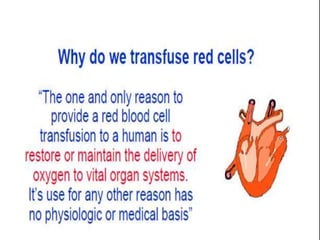
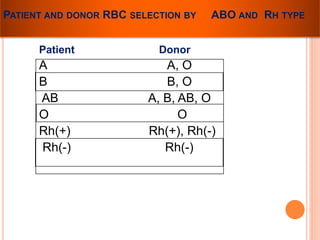
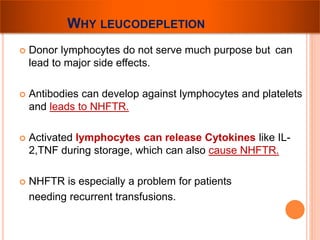
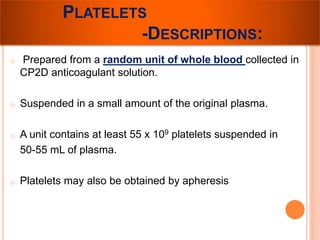
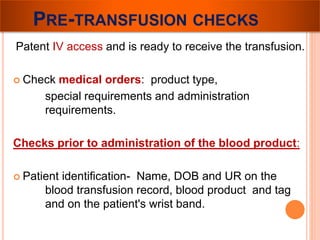
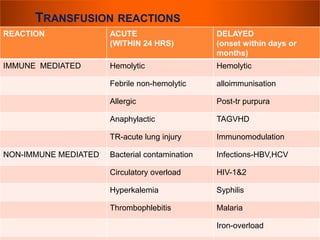
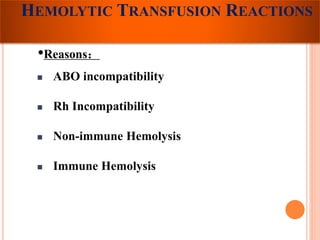
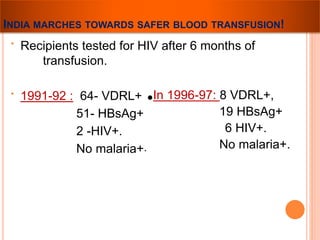
The document provides a history of blood transfusion from early attempts in 1492 when the blood of three boys was used in an unsuccessful transfusion for Pope Innocent VIII, to Richard Lower's first successful animal-to-animal transfusion in 1665. It discusses the development of using animal blood for human transfusion and the progression to modern component therapy. Key events and discoveries regarding donor selection criteria, screening tests, blood typing, storage of blood products, and the use of platelet and plasma components are summarized.