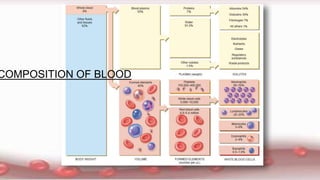
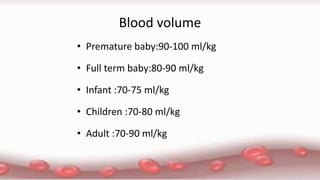
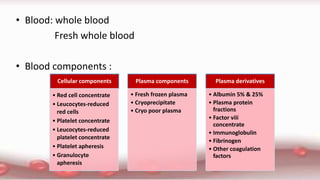
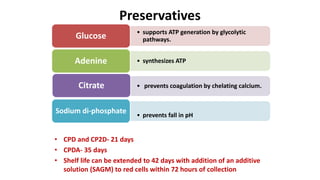
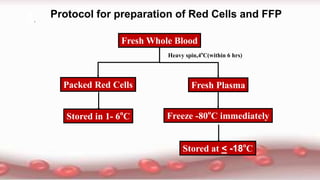
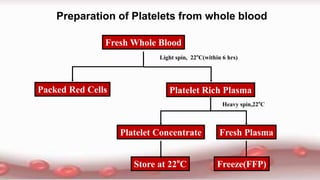
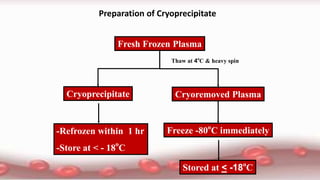
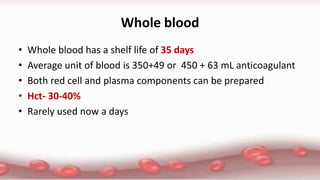
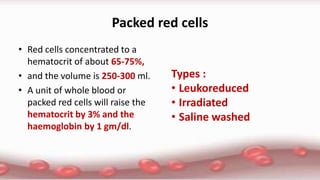
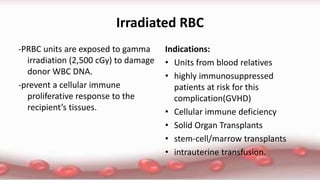
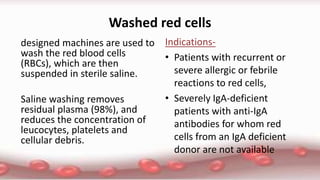
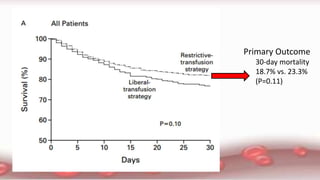
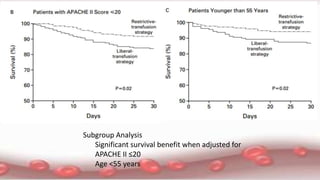
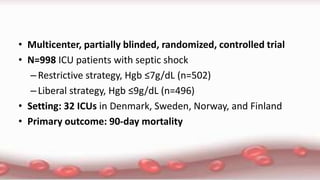
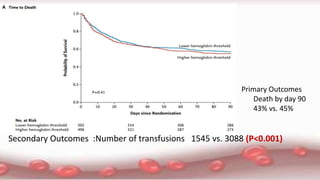
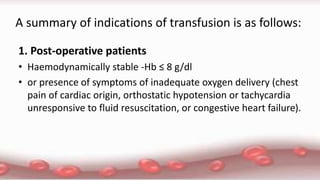
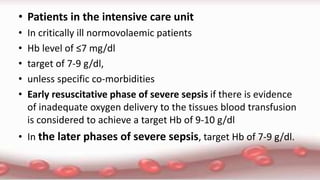
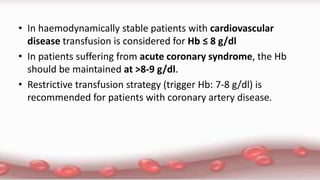
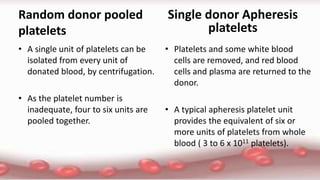
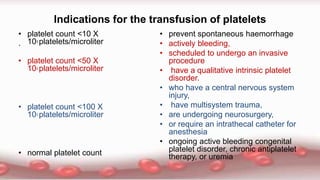
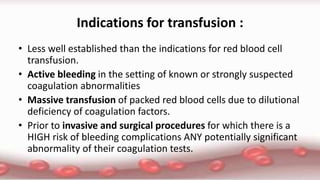
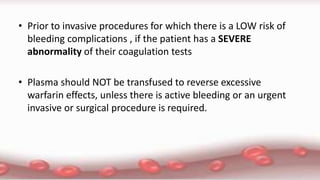
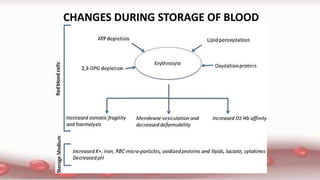
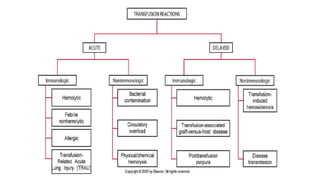
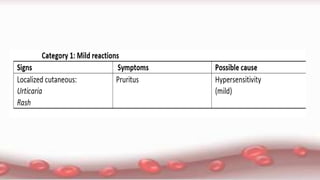
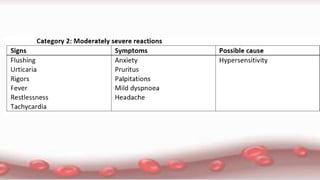
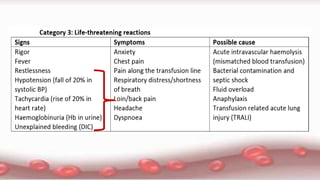
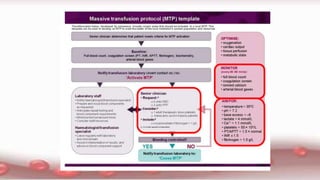
This document provides an overview of blood component therapy. It discusses the composition of blood and history of blood transfusion. It describes the preparation of various blood components like red blood cells, platelets, plasma, and cryoprecipitate. It outlines the indications and guidelines for transfusion of these components. It also reviews trials on restrictive versus liberal transfusion strategies and discusses adverse effects and management of transfusion reactions.