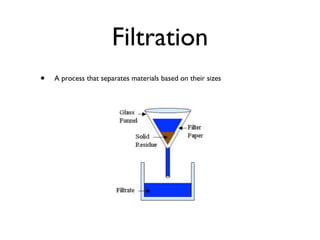
There are currently 118 known elements that make up all matter. Elements are pure substances that contain only one type of atom, while compounds are made of two or more elements or other compounds. Mixtures have a variable composition because their ingredients are not uniformly distributed.