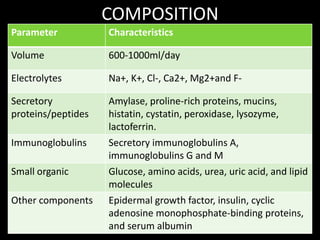
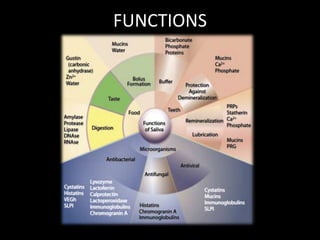
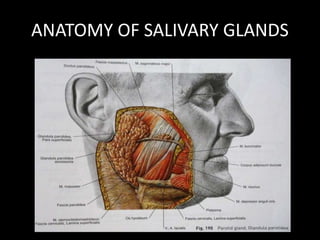
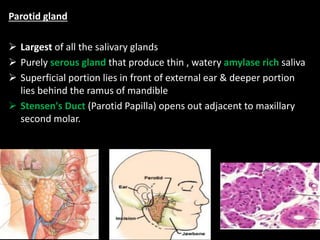
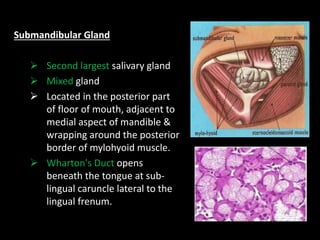
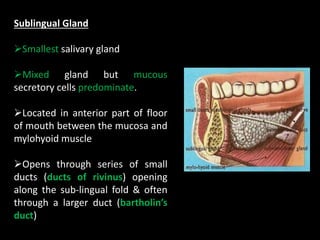
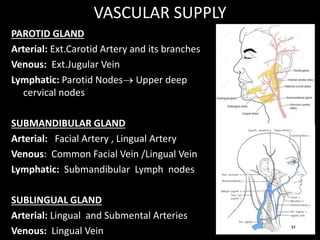
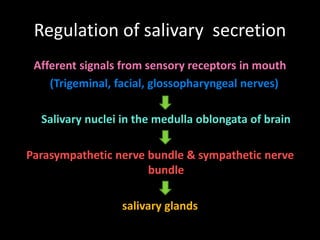
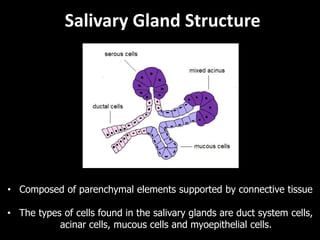
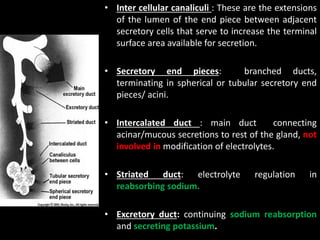
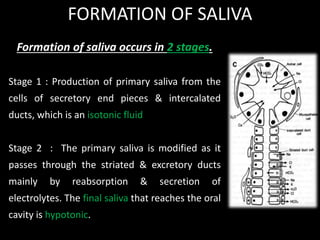
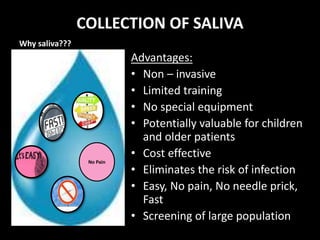
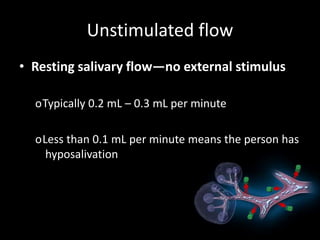
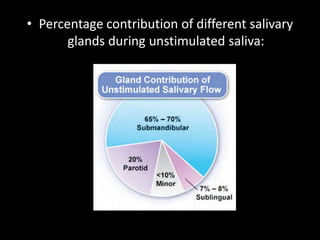
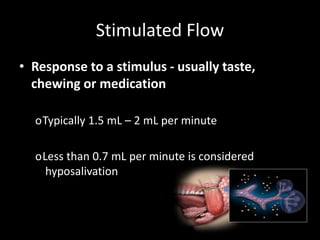
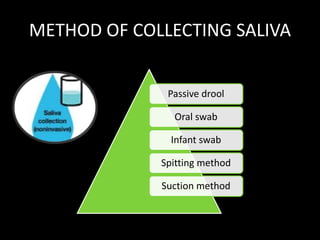
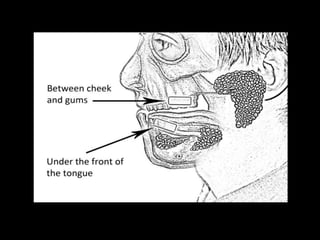
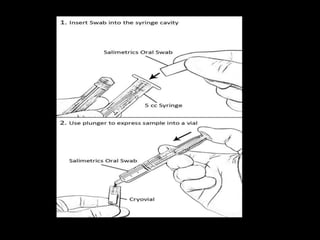
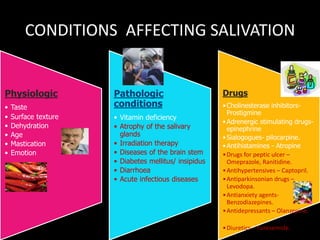
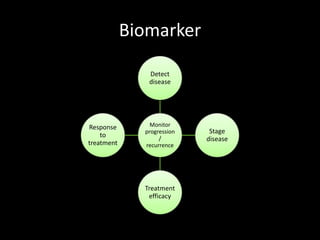
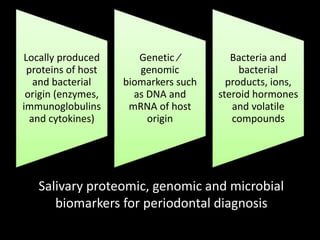
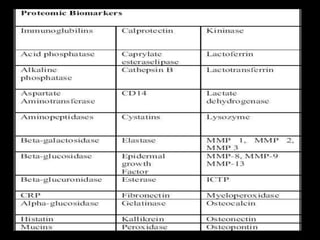
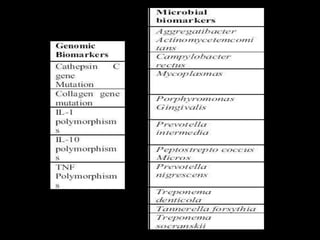
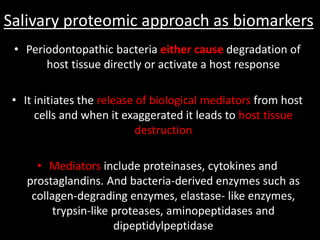
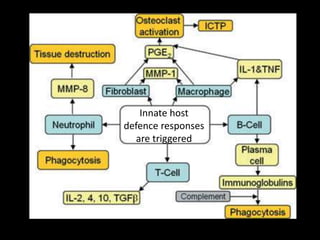
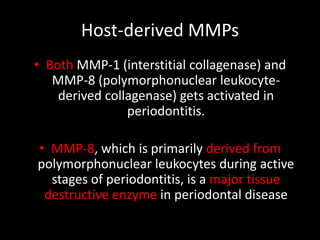
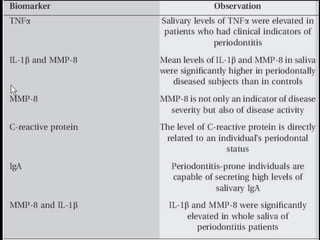
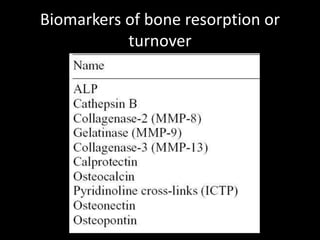
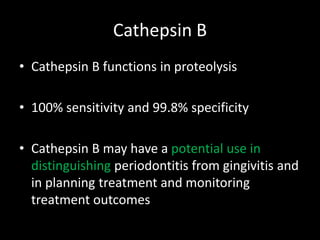
This document provides an overview of a presentation on salivary glands. It discusses the classification, composition, functions, and anatomy of salivary glands. It also covers the formation and collection of saliva, conditions affecting salivation, salivary markers for periodontal diagnosis, roles of salivary enzymes and hormones, and the potential for saliva as a future diagnostic fluid. Biomarkers in saliva are classified and salivary substitutes are briefly mentioned.