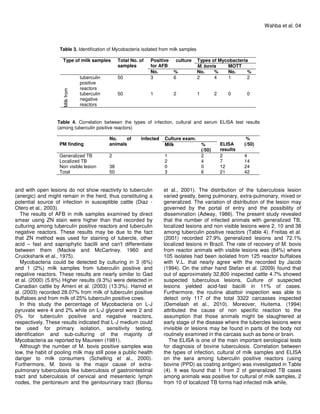
This document summarizes a study that detected bovine tuberculosis in milk and serum samples from dairy farm animals in Assiut City, Egypt. Several methods were used for detection, including the tuberculin skin test, microscopic examination using Ziehl-Neelsen staining, bacterial culture using Lowenstein Jensen media, and an ELISA test using bovine PPD as the coating antigen. Acid-fast bacilli were detected microscopically in 7% of milk samples from tuberculin-positive reactors and 3% from tuberculin-negative reactors. Mycobacteria were isolated via culture from 3-4% of milk samples from tuberculin-positive reactors and 1-2% from negative reactors