Embed presentation
Download to read offline
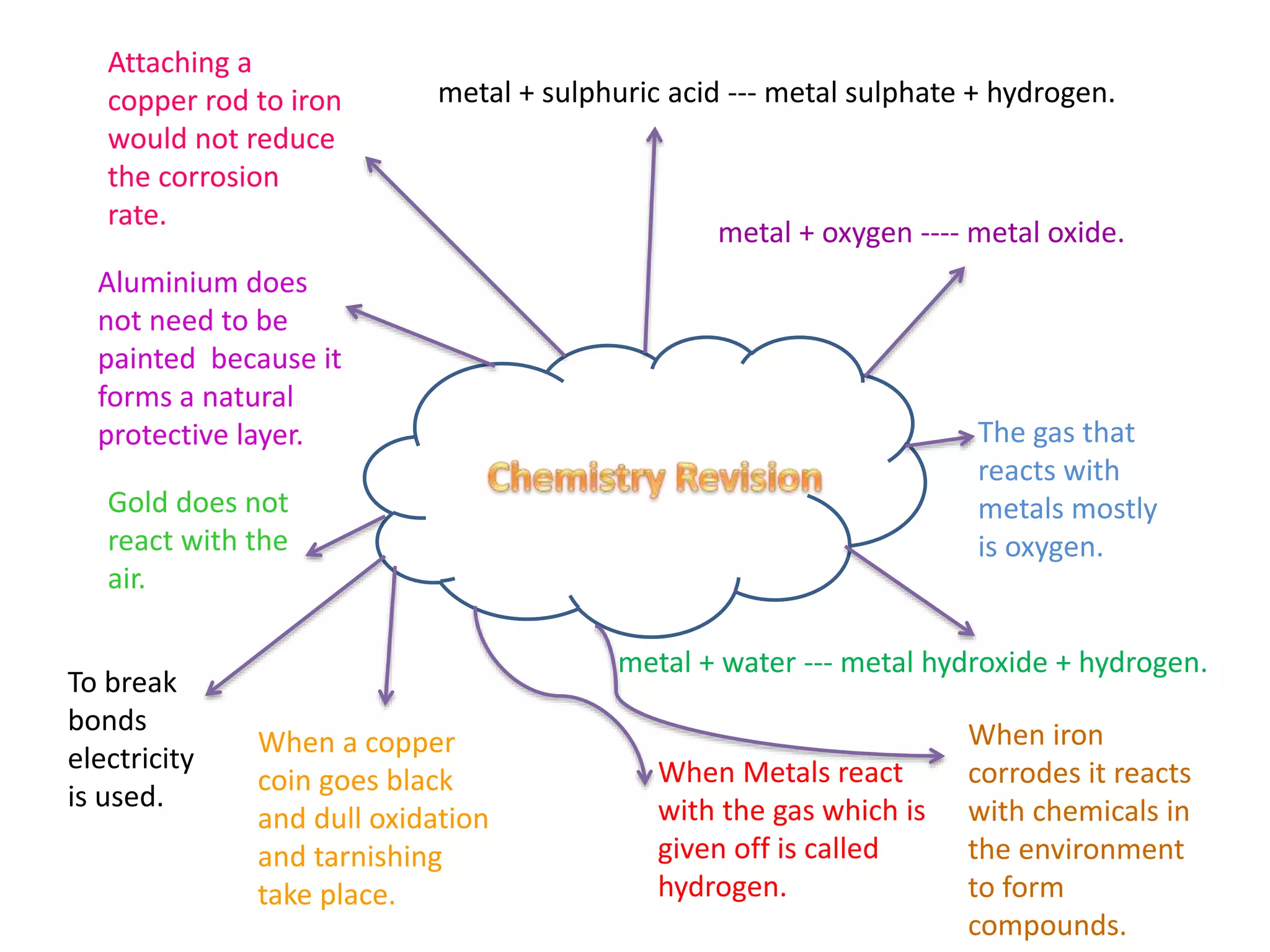

Metals react with oxygen, sulfuric acid, and water to form metal oxides, sulfates, and hydroxides respectively, with hydrogen gas given off as a product. Aluminum forms a protective oxide layer preventing further corrosion without needing paint, while gold does not react with air at all, and attaching copper to iron does not reduce the corrosion rate of iron which forms compounds when reacting with chemicals in its environment through oxidation and tarnishing processes.
