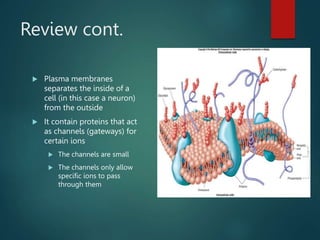
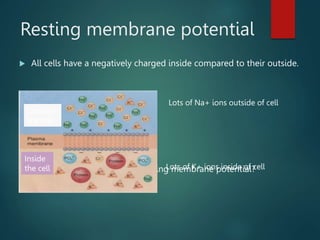
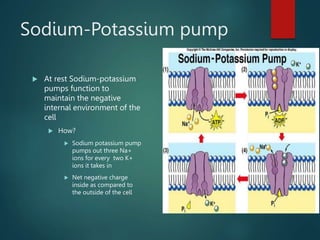
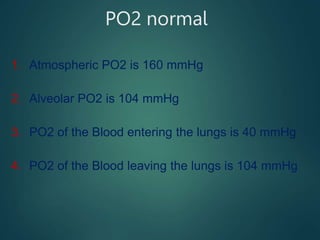
This document summarizes several key physiological concepts related to high altitude physiology:
1. At high altitudes, the lower atmospheric pressure results in lower oxygen levels in the blood (hypoxemia). The body responds through acclimatization mechanisms like increased respiration and red blood cell production.
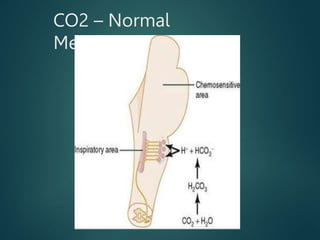
2. Initially, the low oxygen causes increased breathing while the loss of carbon dioxide inhibits breathing, creating an imbalance. Over time, the kidneys and bone marrow help restore balance.
3. If ascending too quickly, acute mountain sickness can occur from cerebral or pulmonary edema due to the body's inability to properly acclimate to the conditions. Proper acclimatization takes weeks to establish different compensatory mechanisms.