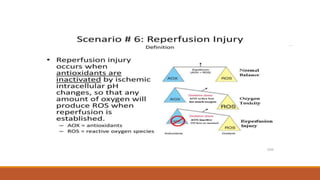
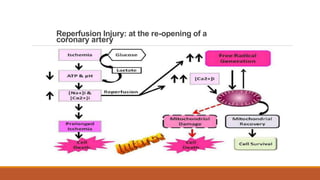
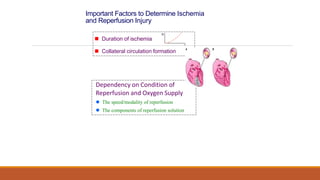
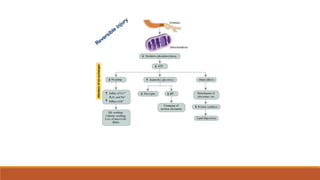
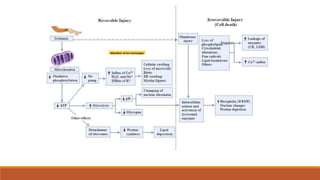
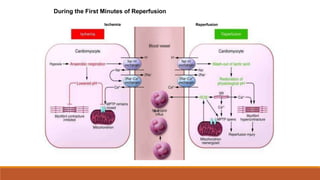
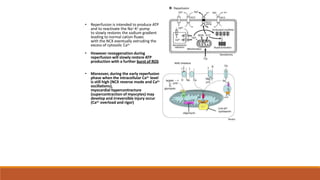
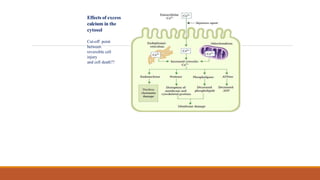
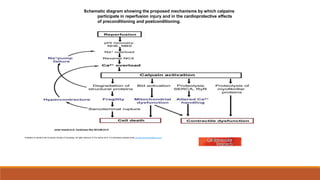
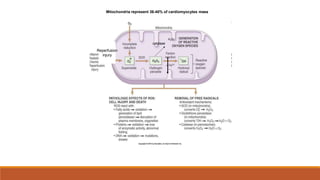
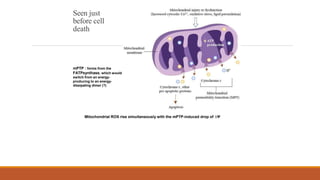
Reperfusion injury occurs after ischemic episodes, where restored blood flow leads to cellular damage due to factors like calcium overload, pH recovery, and reactive oxygen species (ROS) overproduction. Mitochondria play a crucial role in this process, and the injury can exacerbate myocardial infarctions and lead to severe complications such as arrhythmias. Prevention strategies focus on minimizing ischemia and implementing effective reperfusion techniques to protect myocytes during recovery.