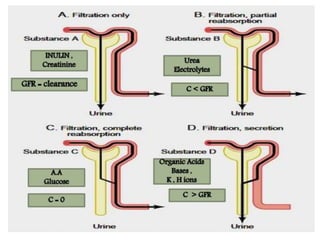
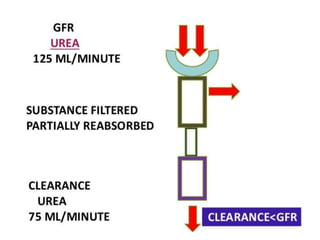
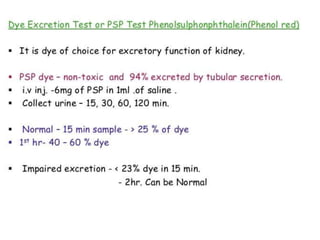
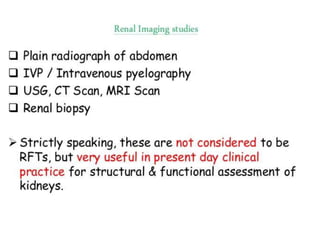
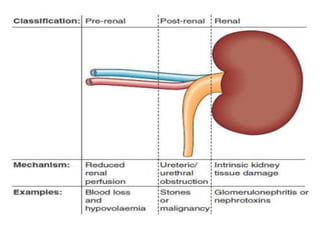
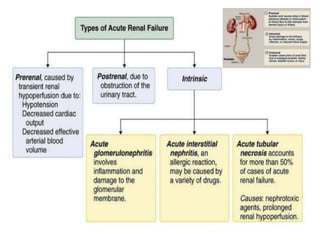
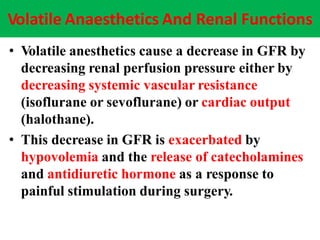
The document discusses renal circulation, regulation of renal blood flow, nephron anatomy and physiology, and effects of various drugs on renal function. The key points are:
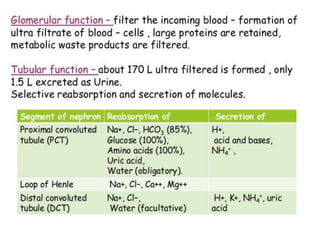
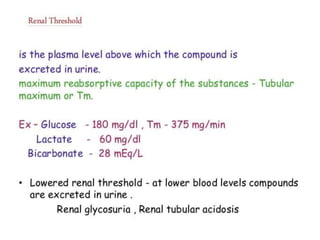
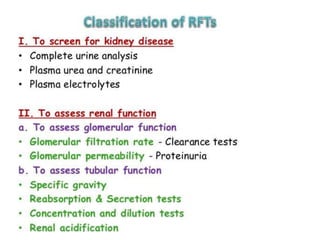
1) The kidneys receive 20-25% of cardiac output despite only weighing 0.4% of body weight. Renal blood flow is regulated by neural, hormonal and local factors to maintain glomerular filtration.
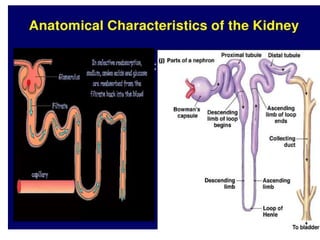
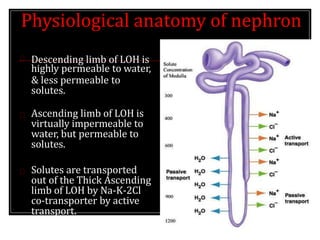
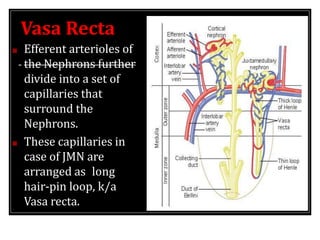
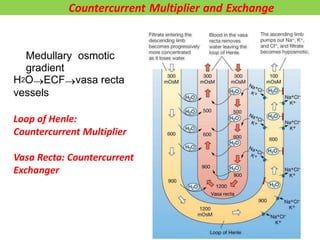
2) Nephrons contain a loop of Henle that acts as a countercurrent multiplier to concentrate urine by establishing an osmotic gradient in the renal medulla, aided by countercurrent exchange in vasa recta capillaries.
3) Drugs like NSAIDs, ACE inhibitors, intravenous agents