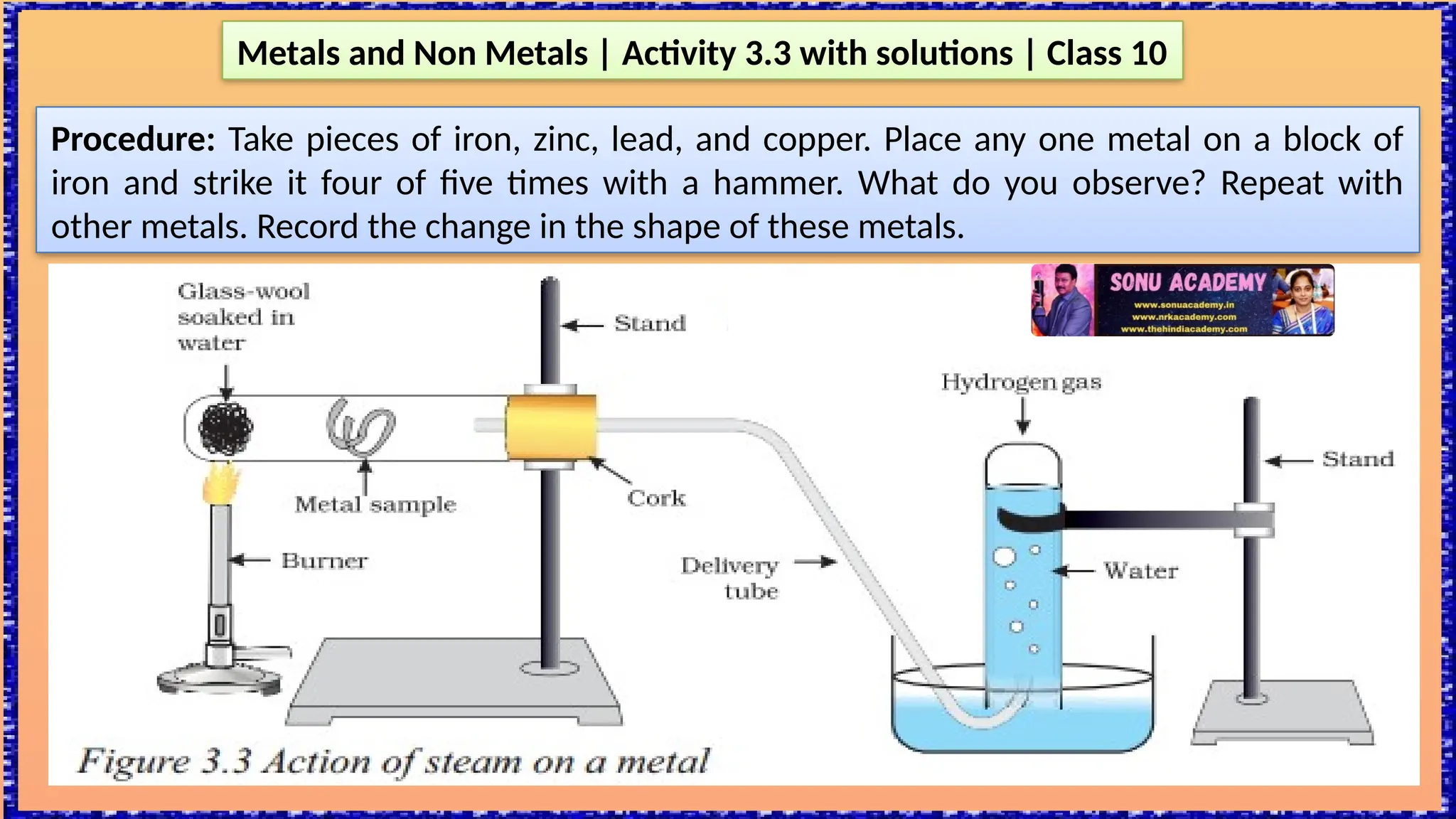
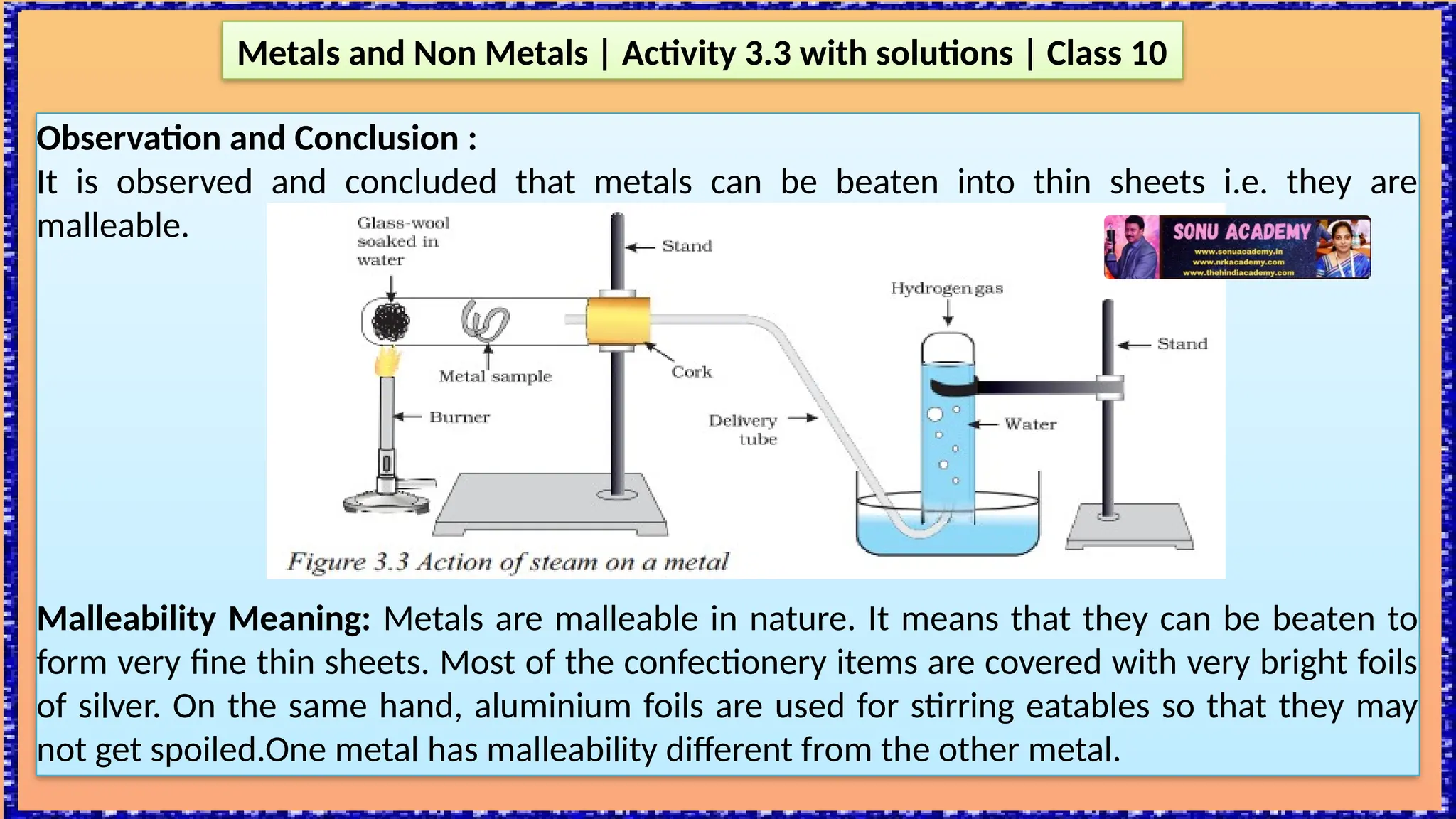
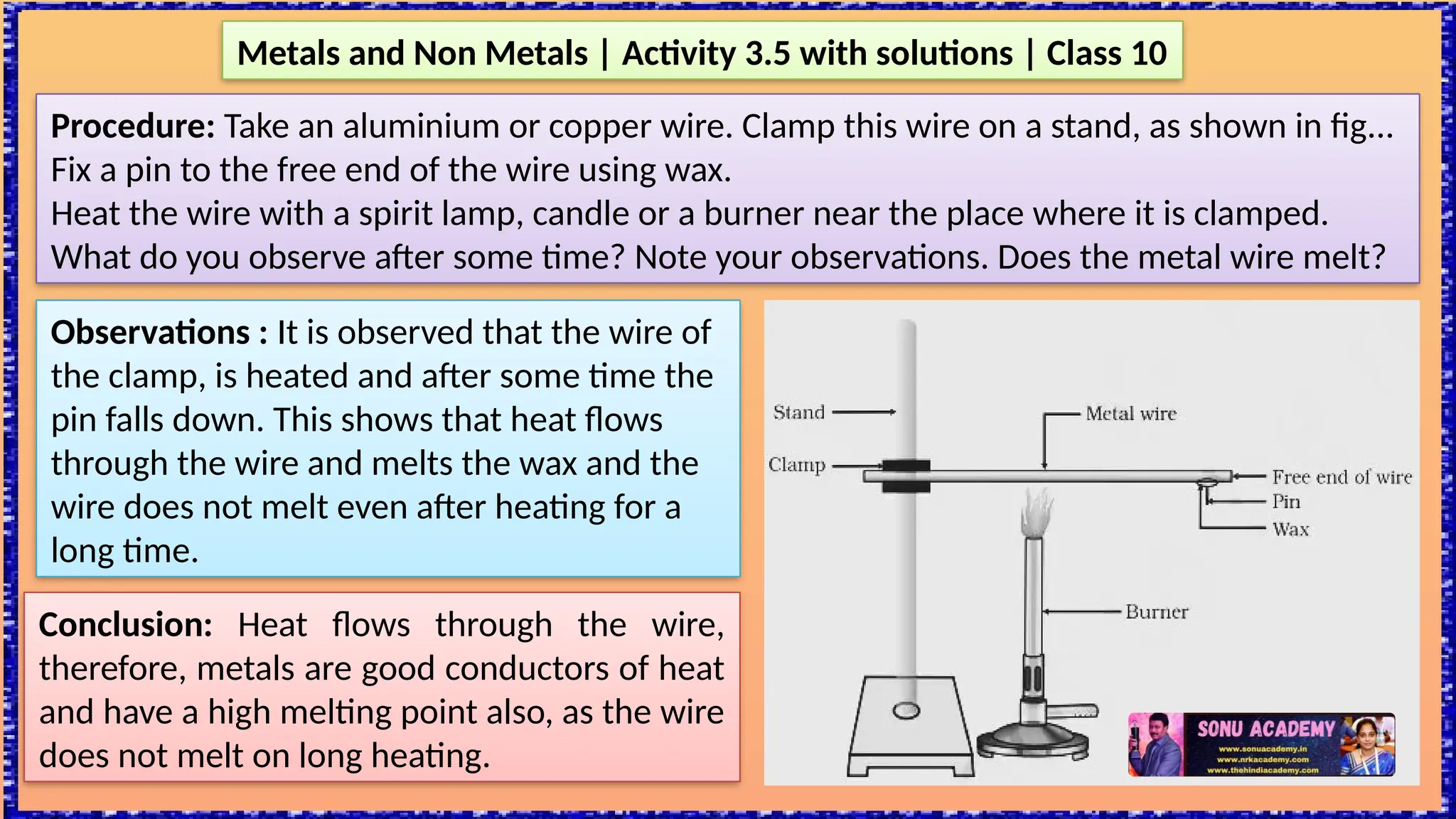
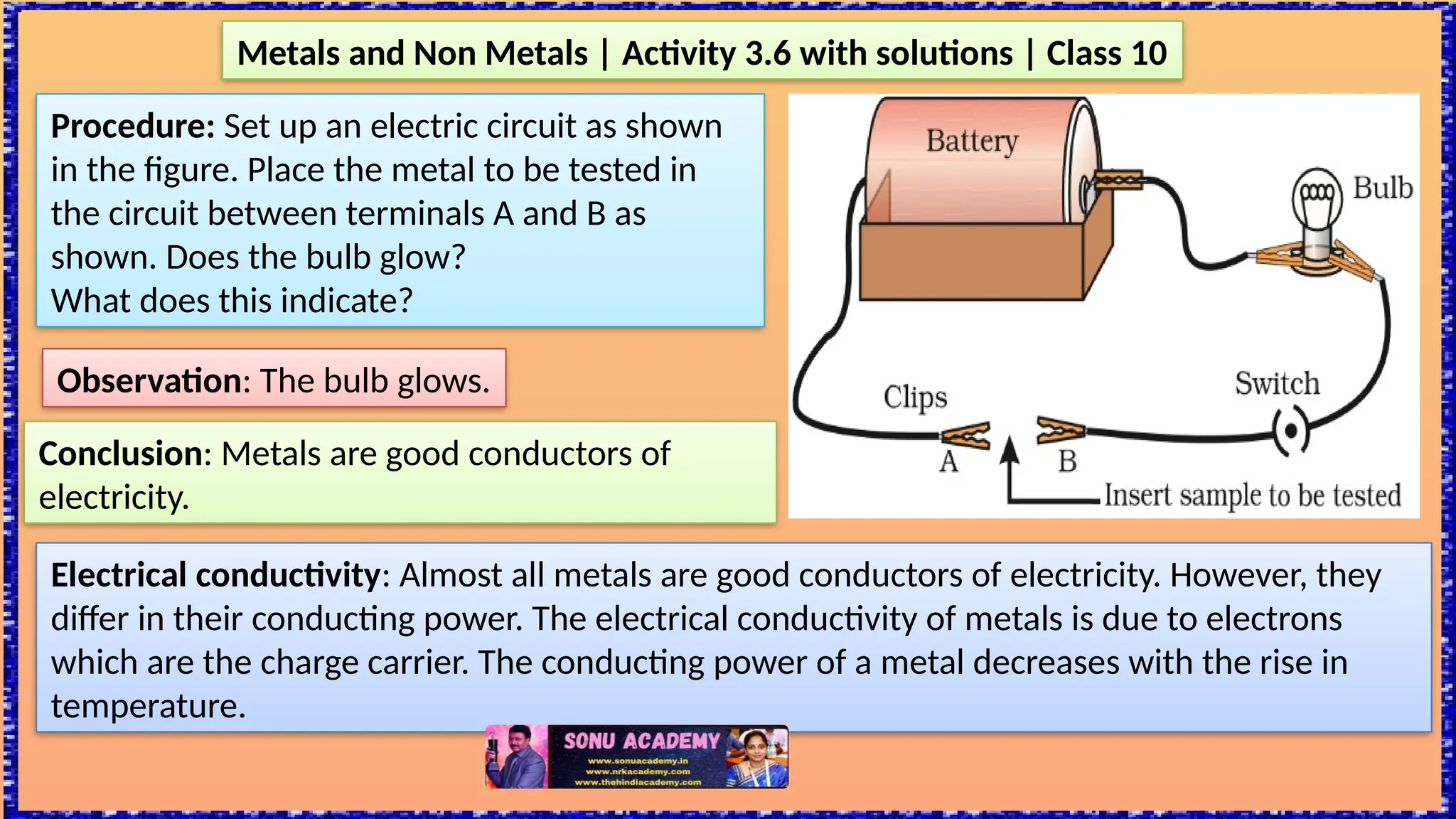
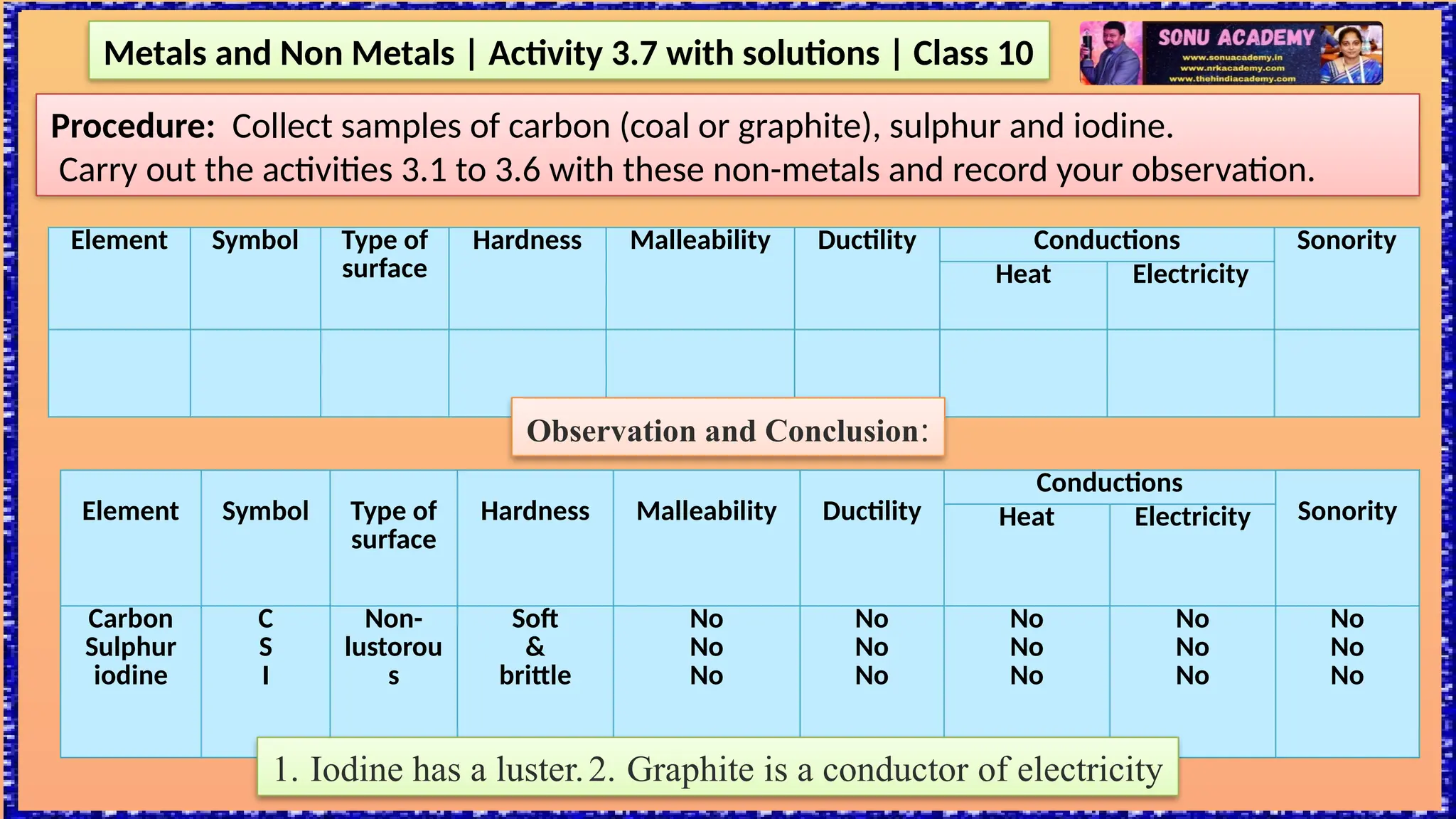
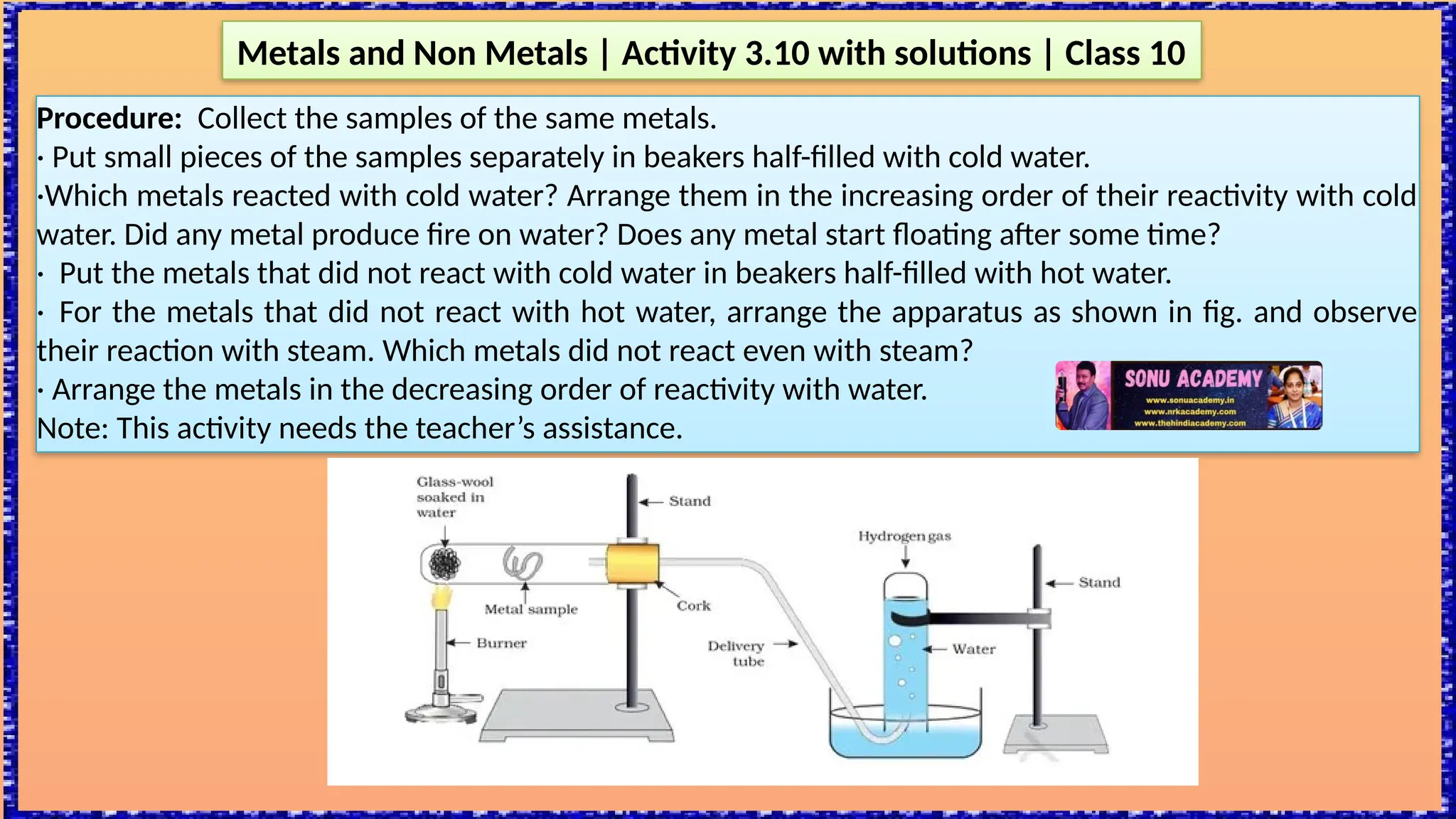
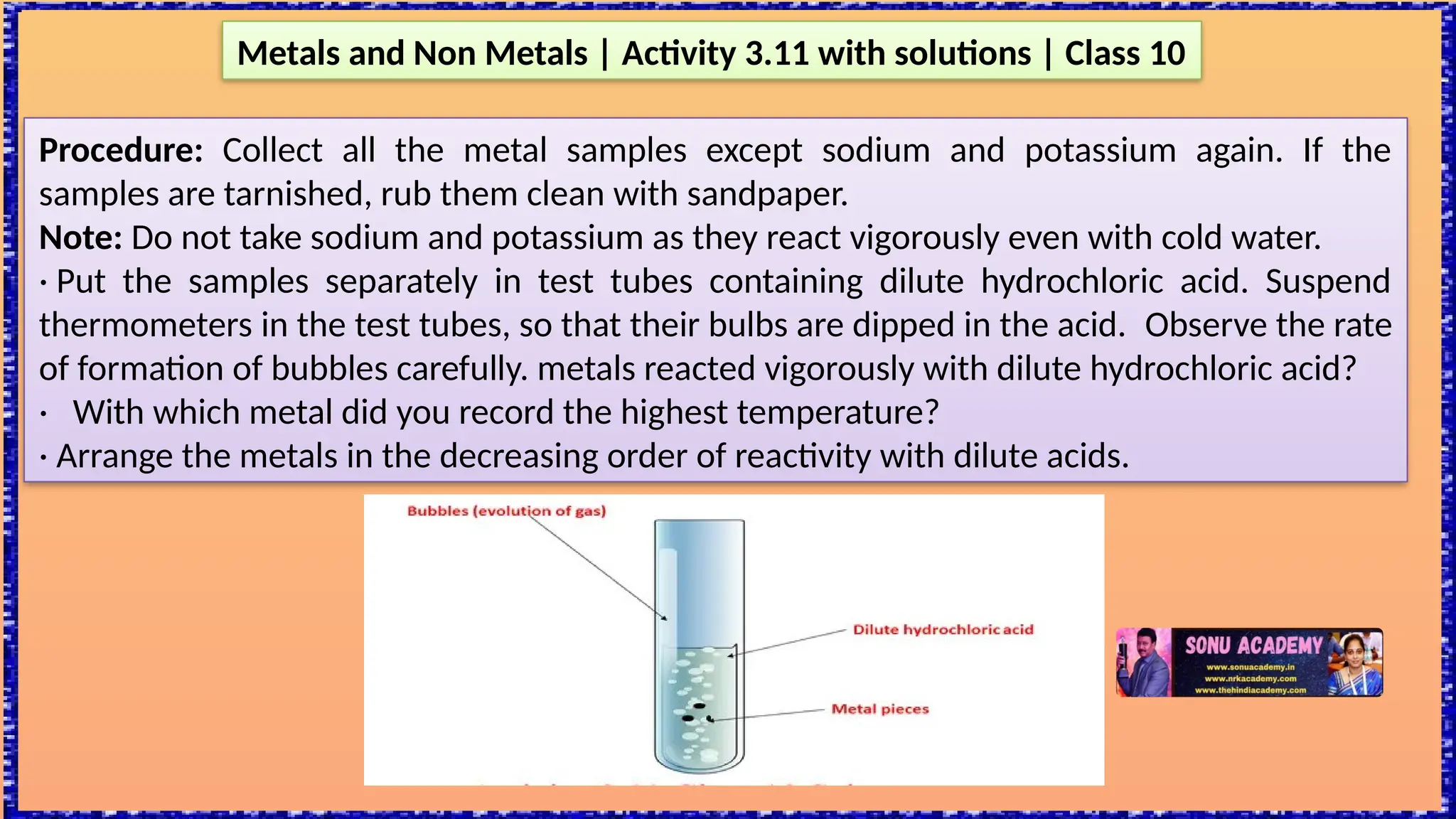
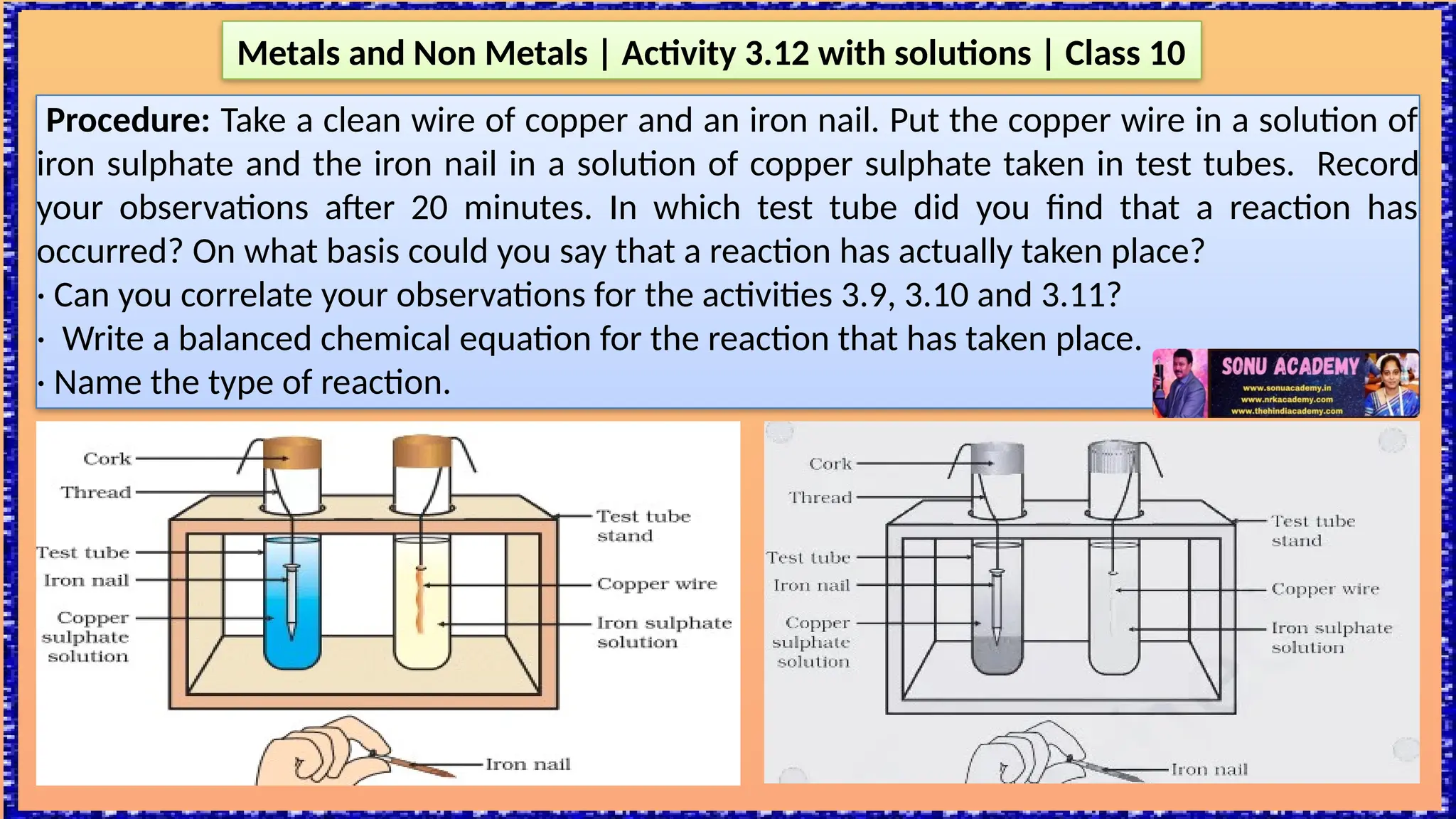
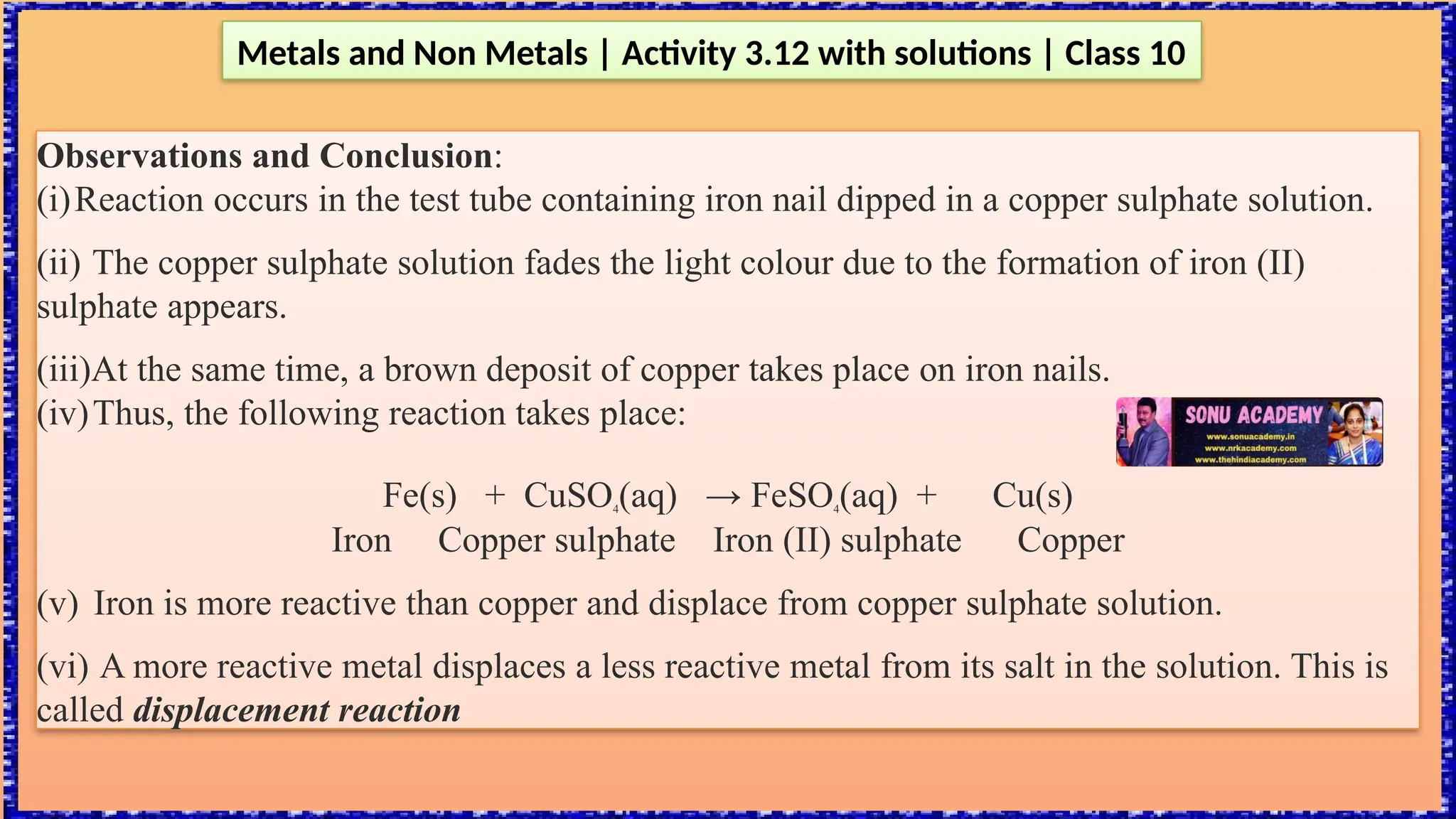
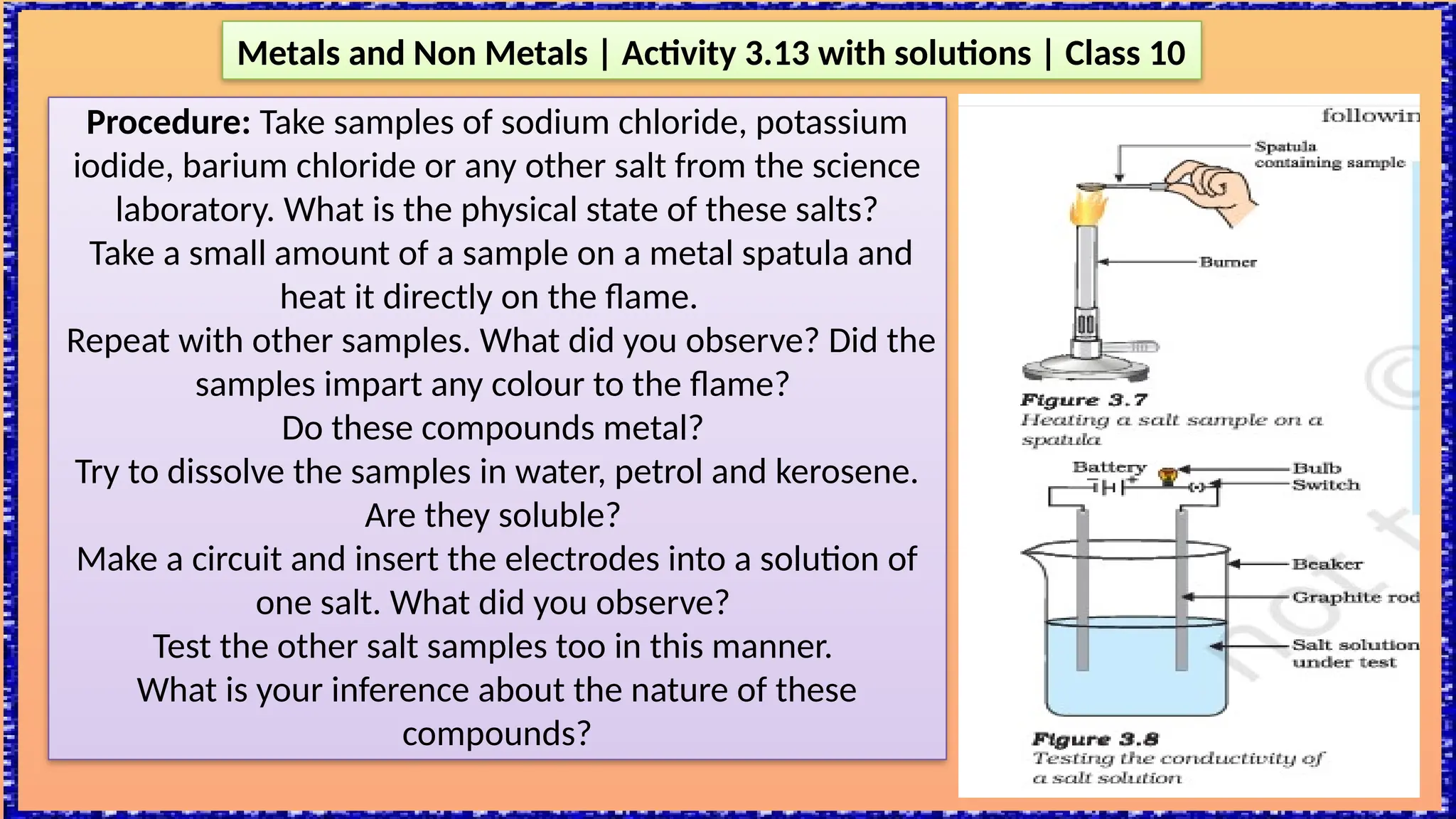
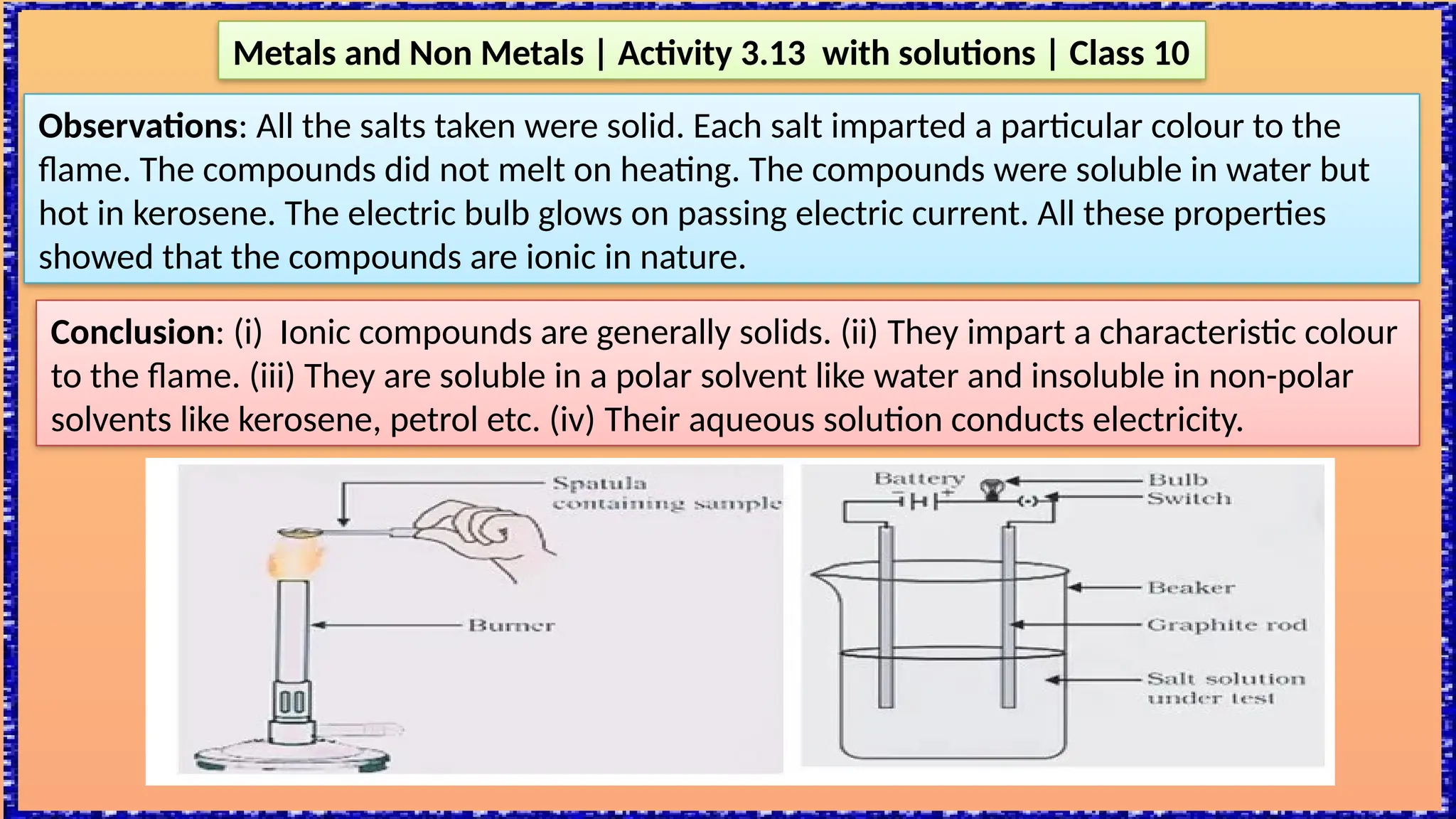
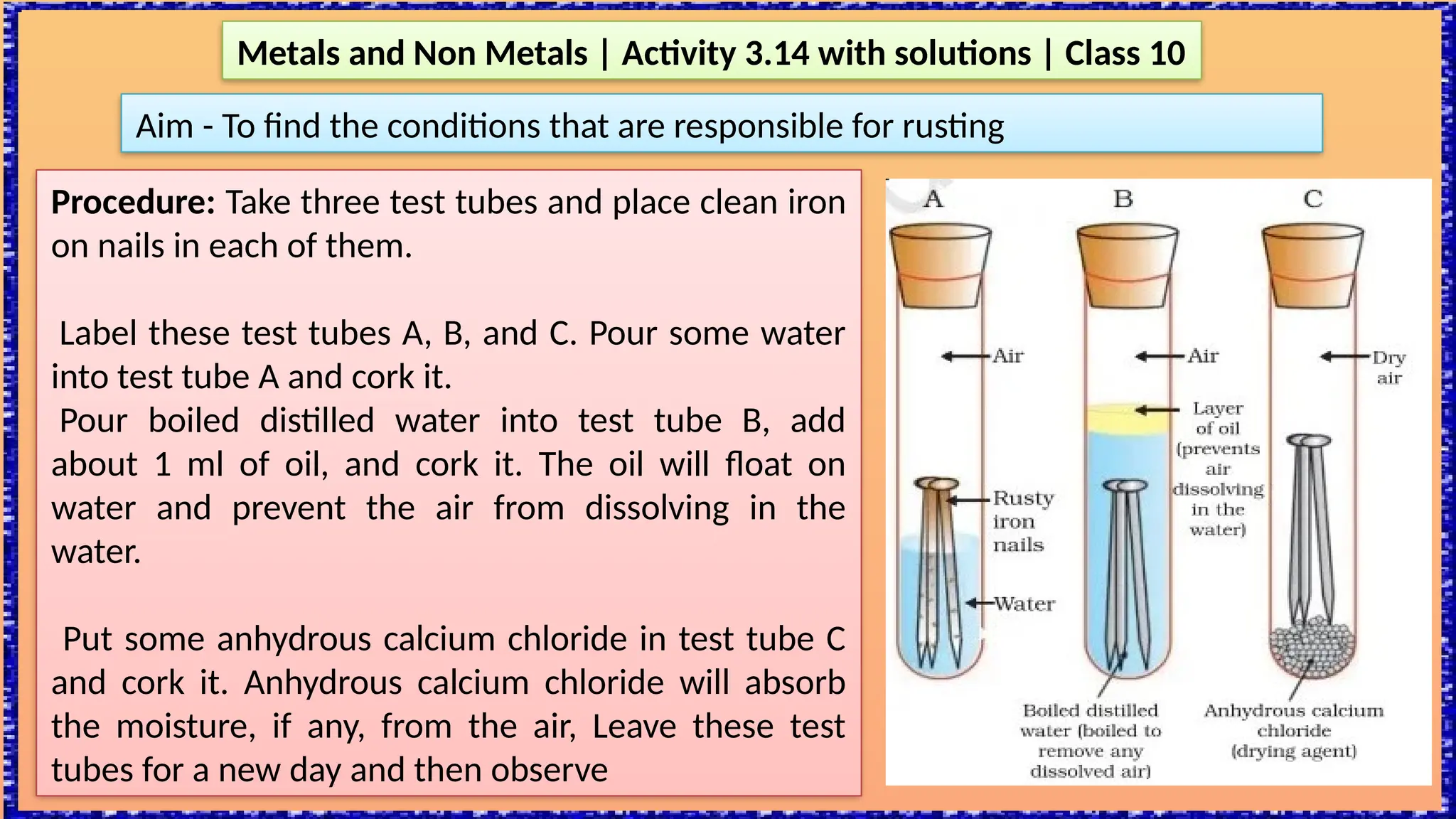
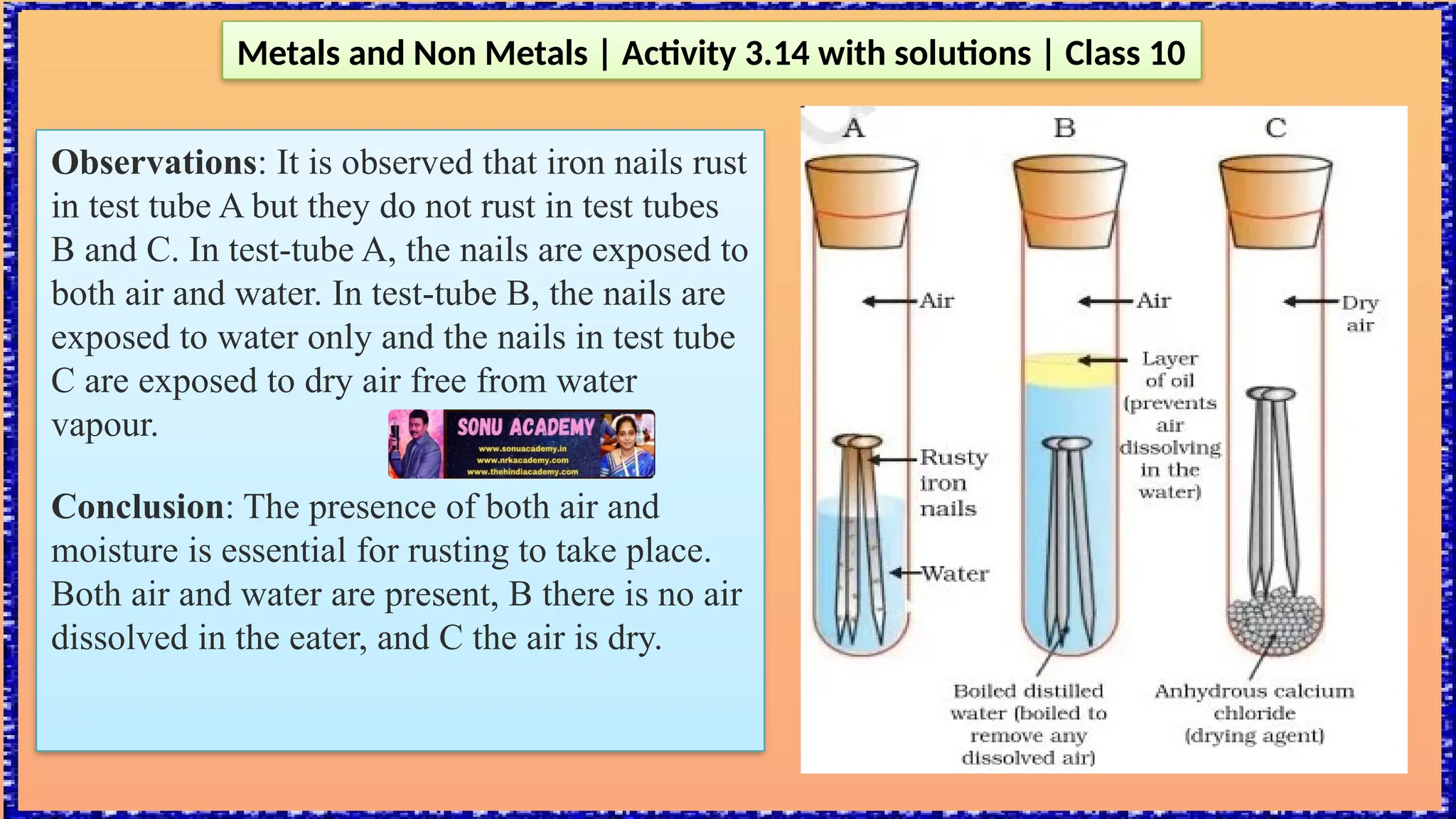
The document outlines various activities for class 10 students to explore the properties of metals and non-metals, including their appearances, hardness, malleability, ductility, thermal and electrical conductivity, reactivity with water and acids, and rusting. Key findings indicate that metals generally possess a shiny appearance, are hard and malleable, good conductors of electricity and heat, while non-metals are typically dull and brittle. The activities also demonstrate the distinction in reactivity between different metals and the formation of metal oxides, noting that metallic oxides are basic and non-metal oxides are acidic.