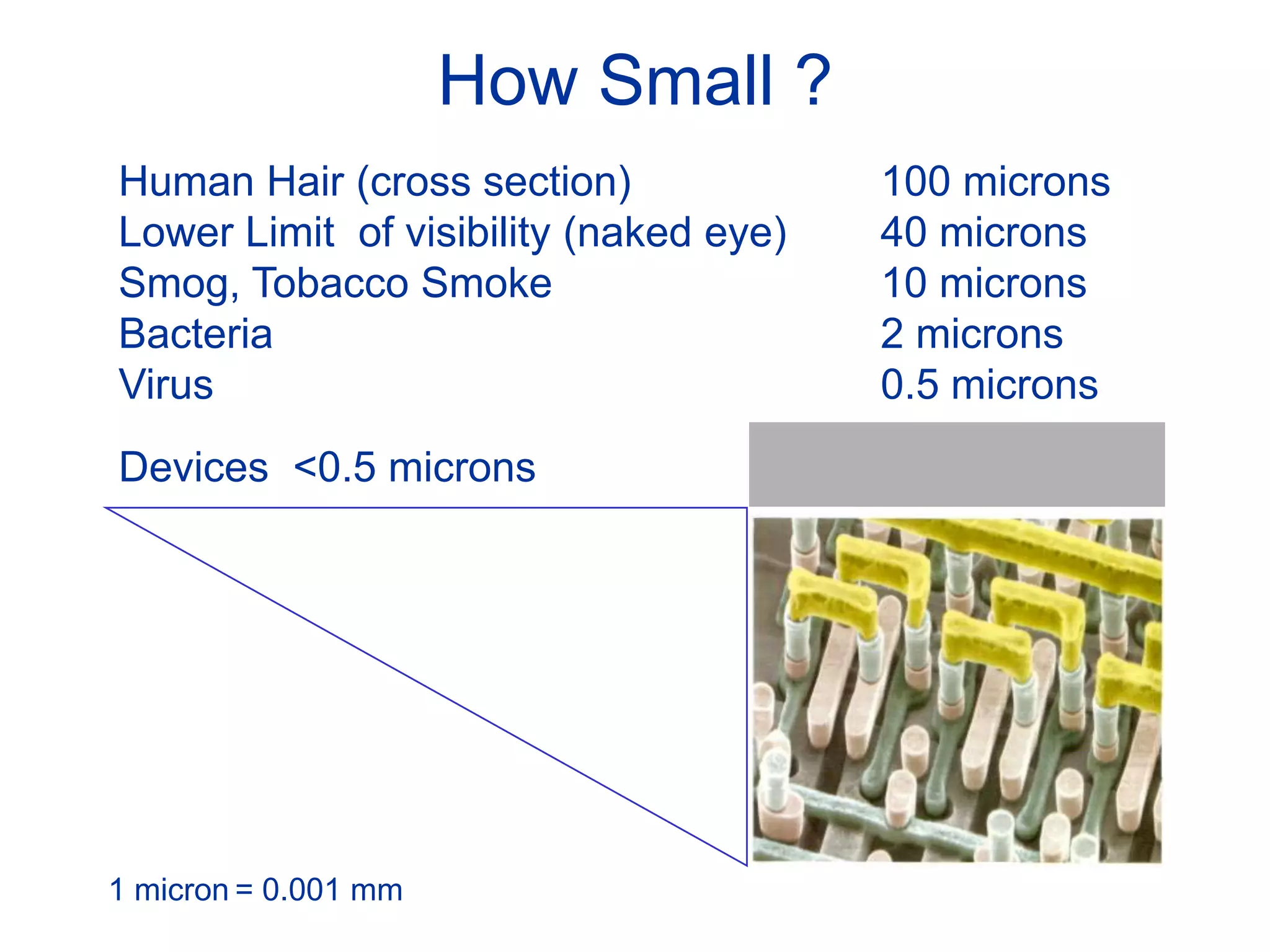
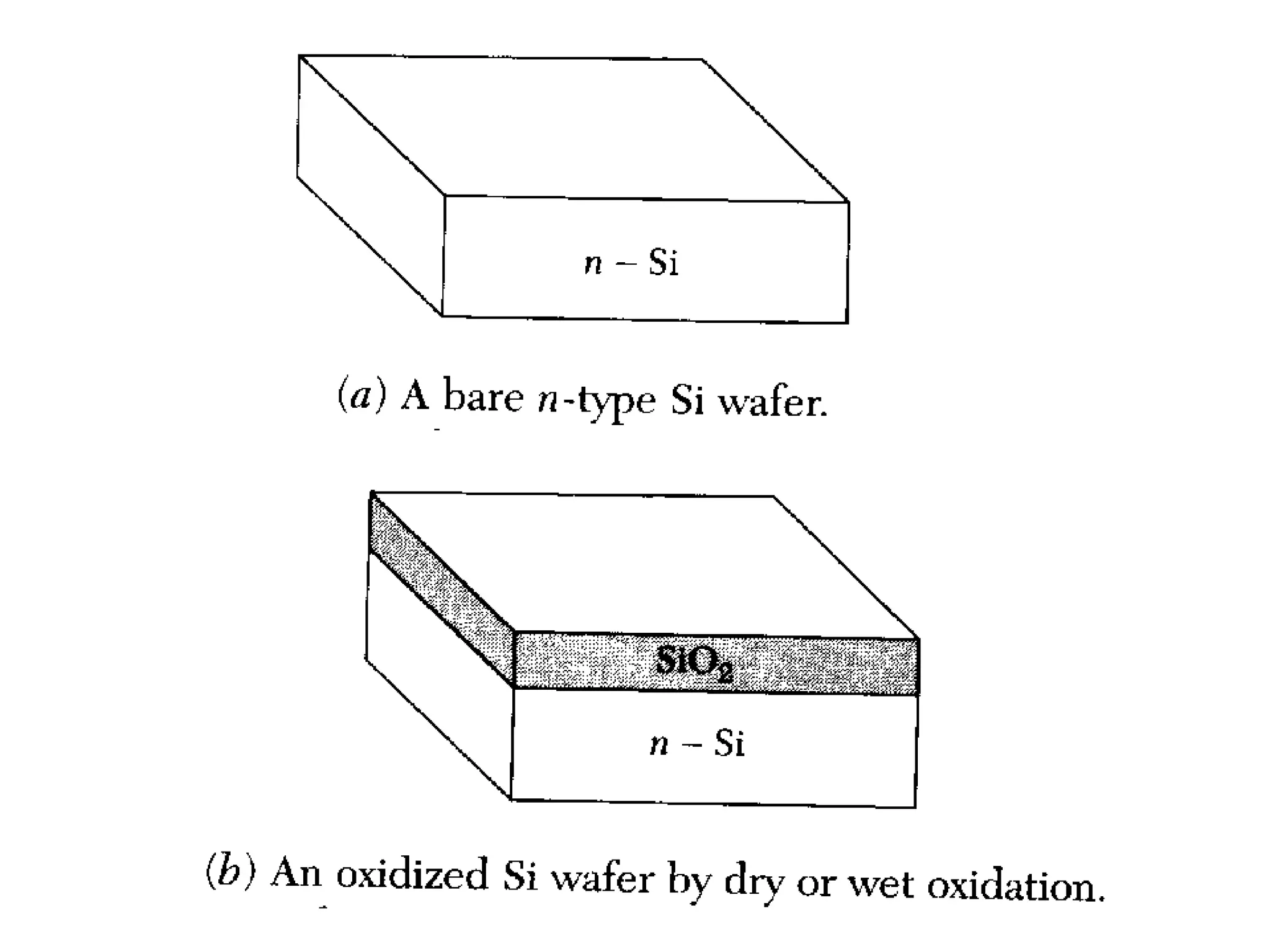
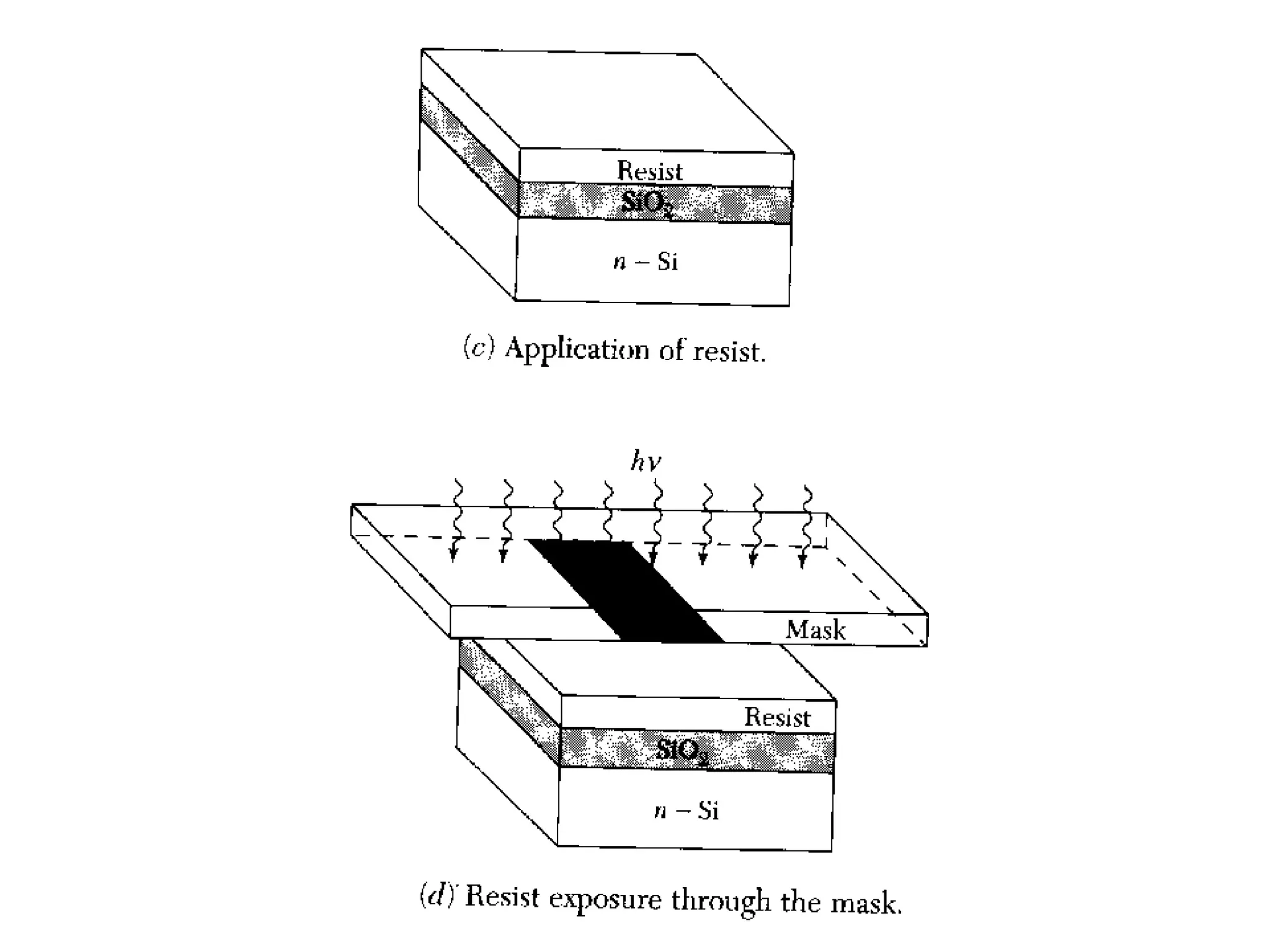
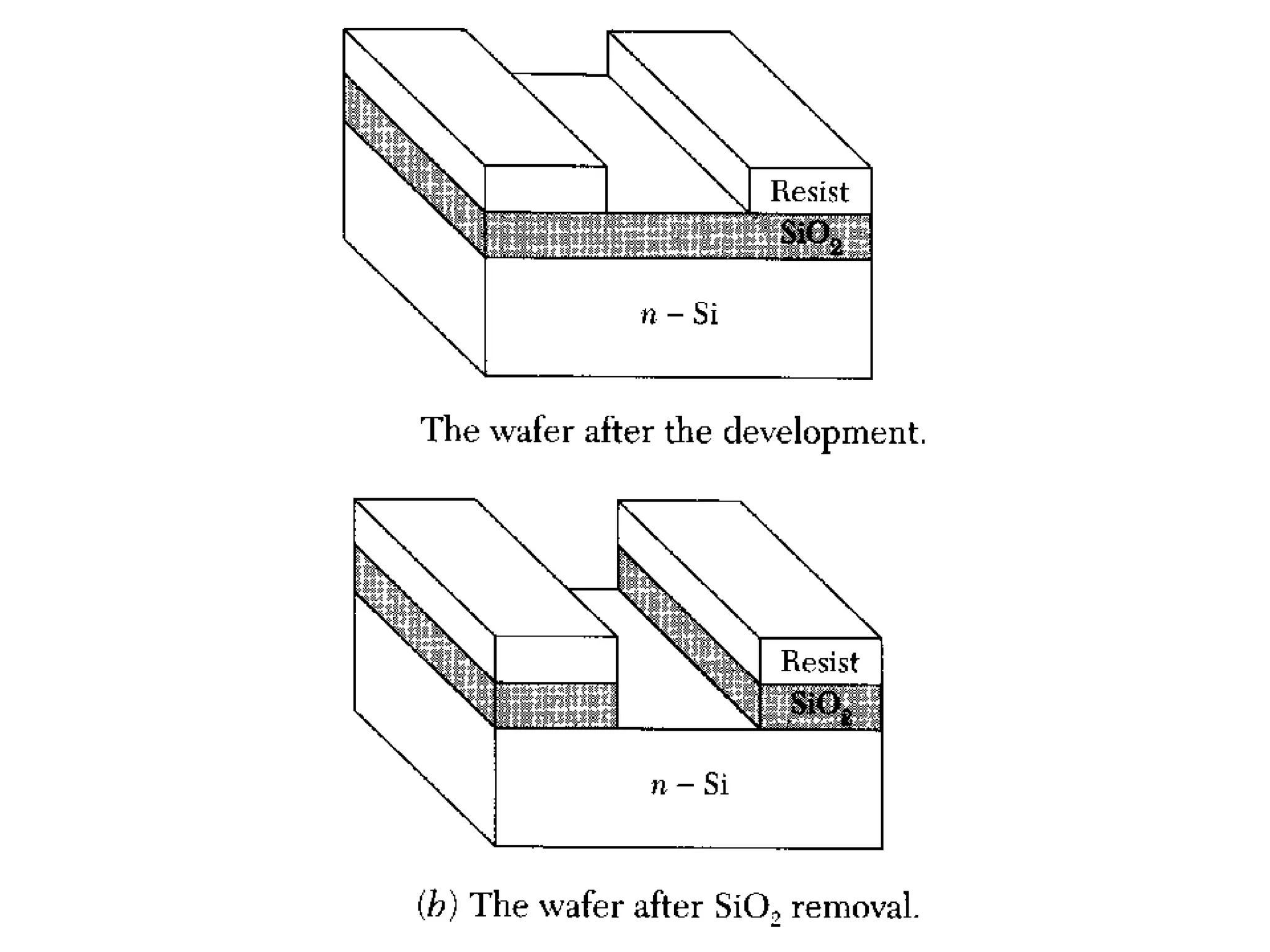
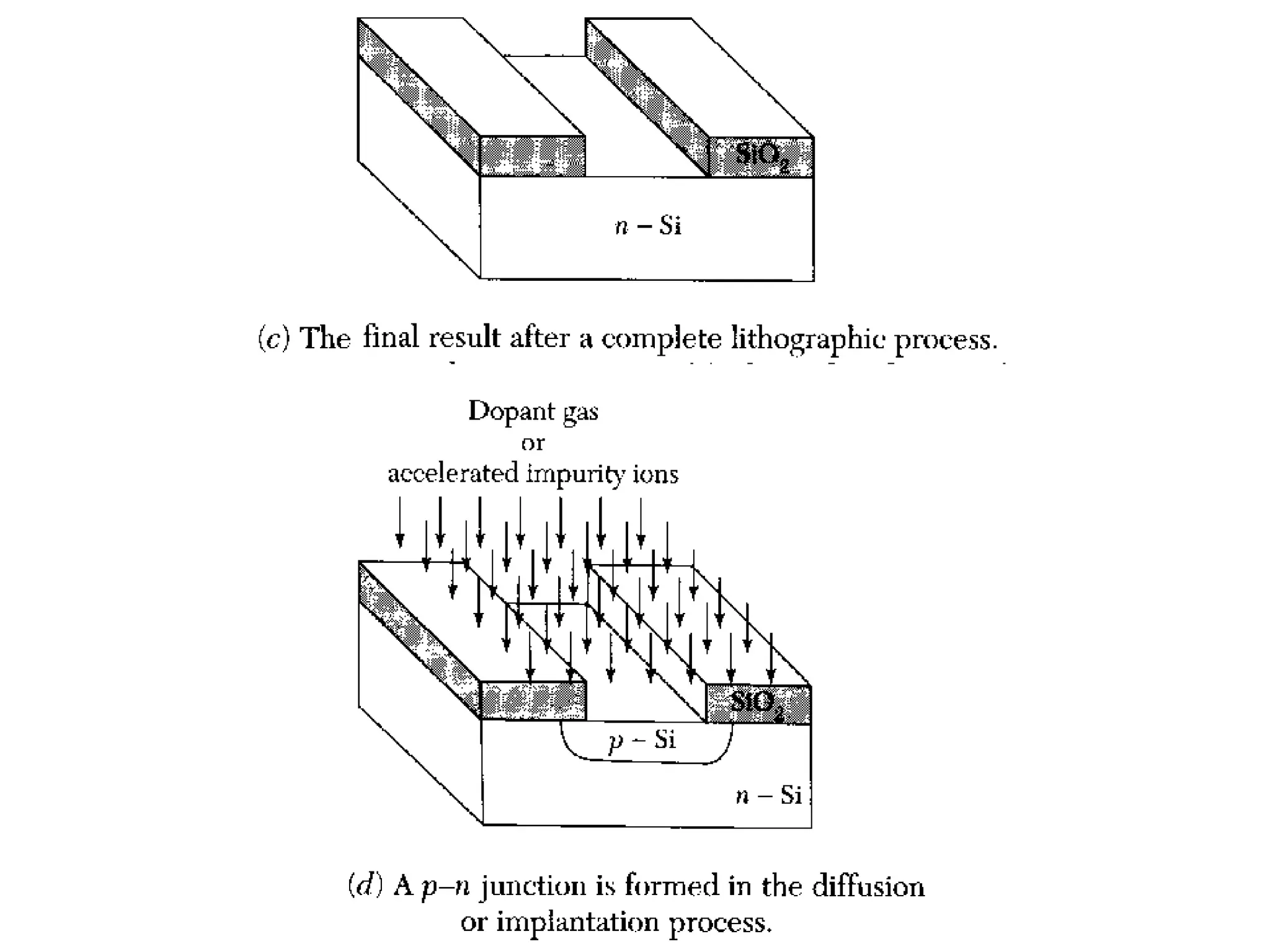
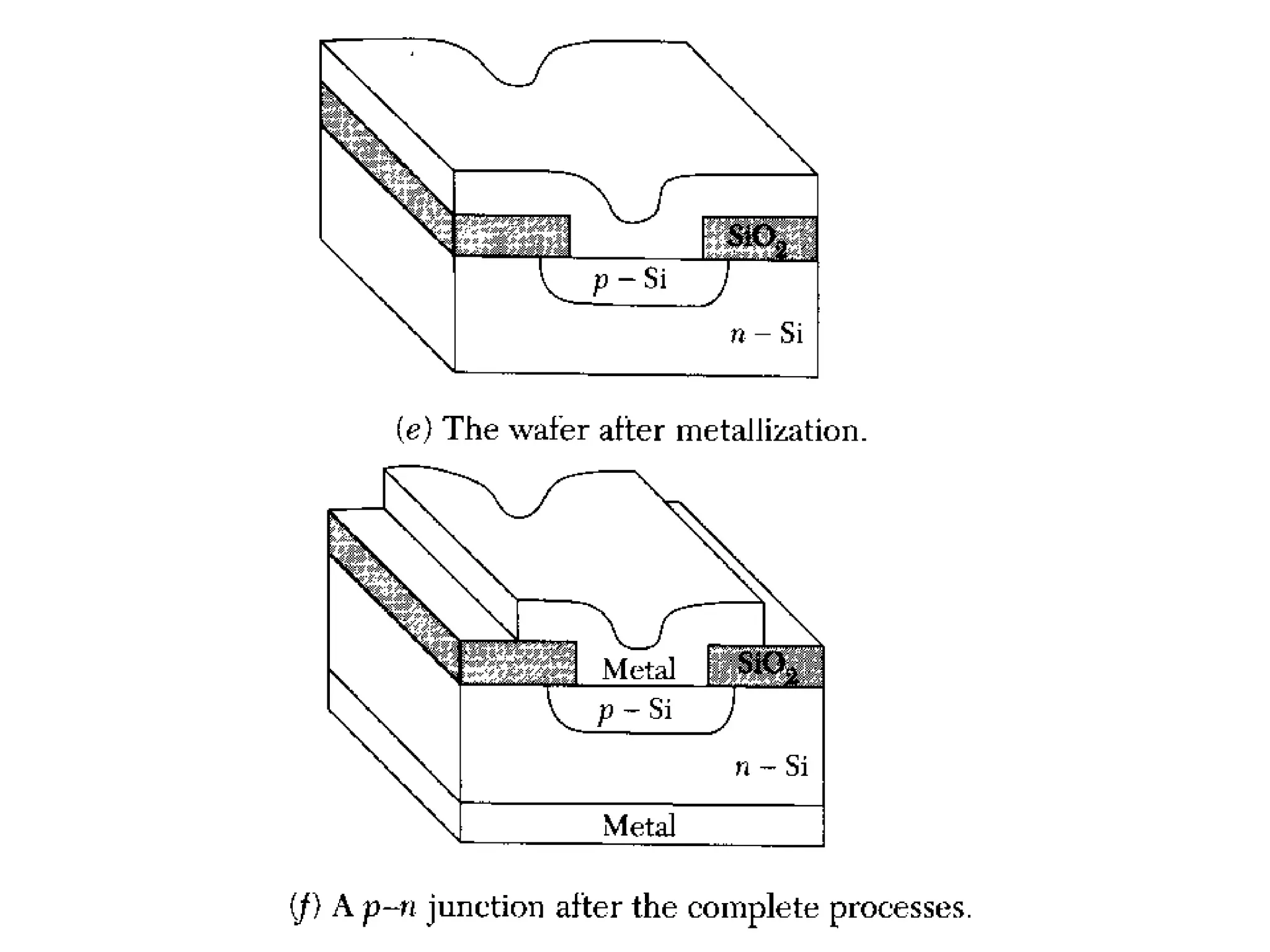
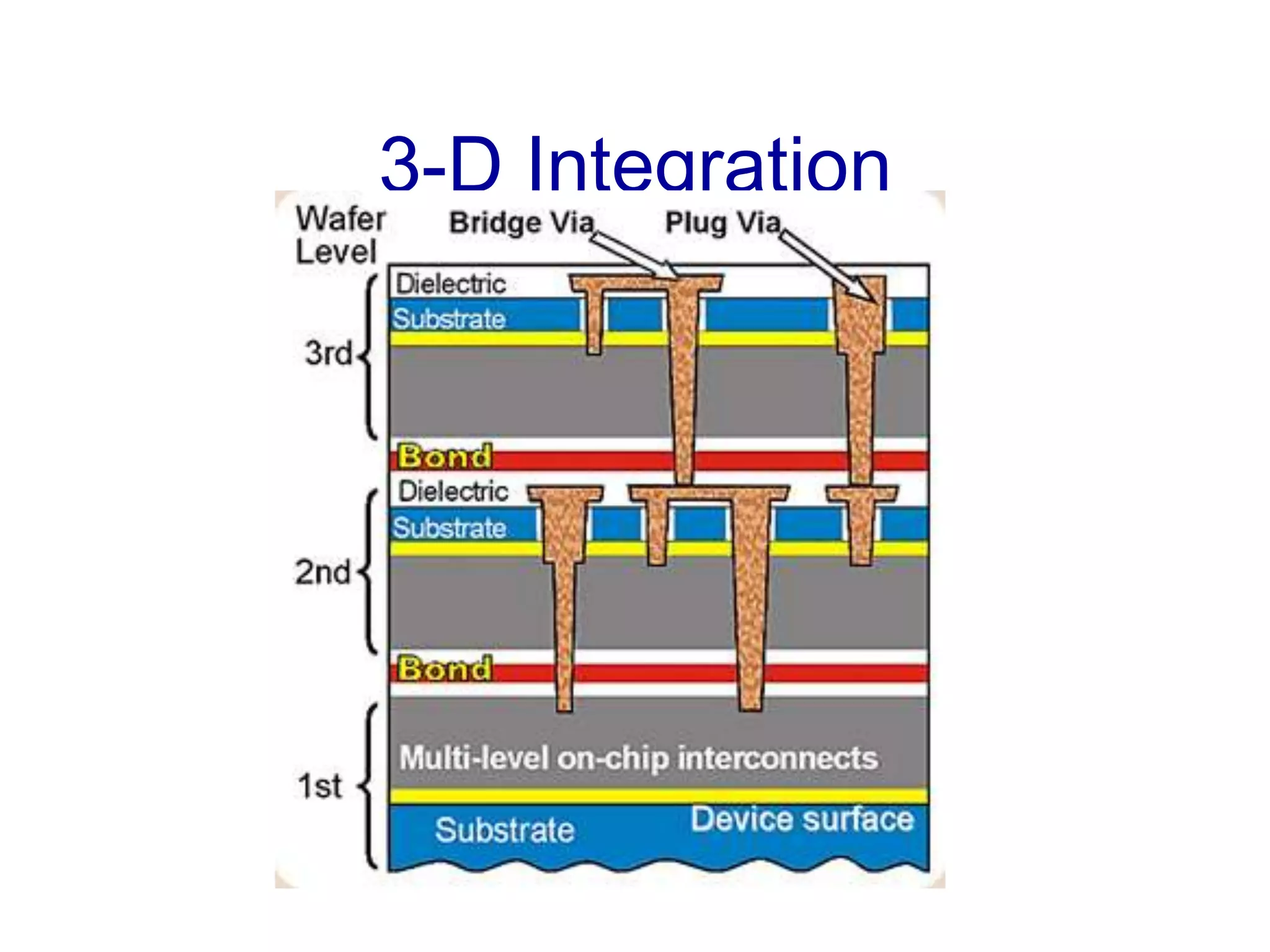
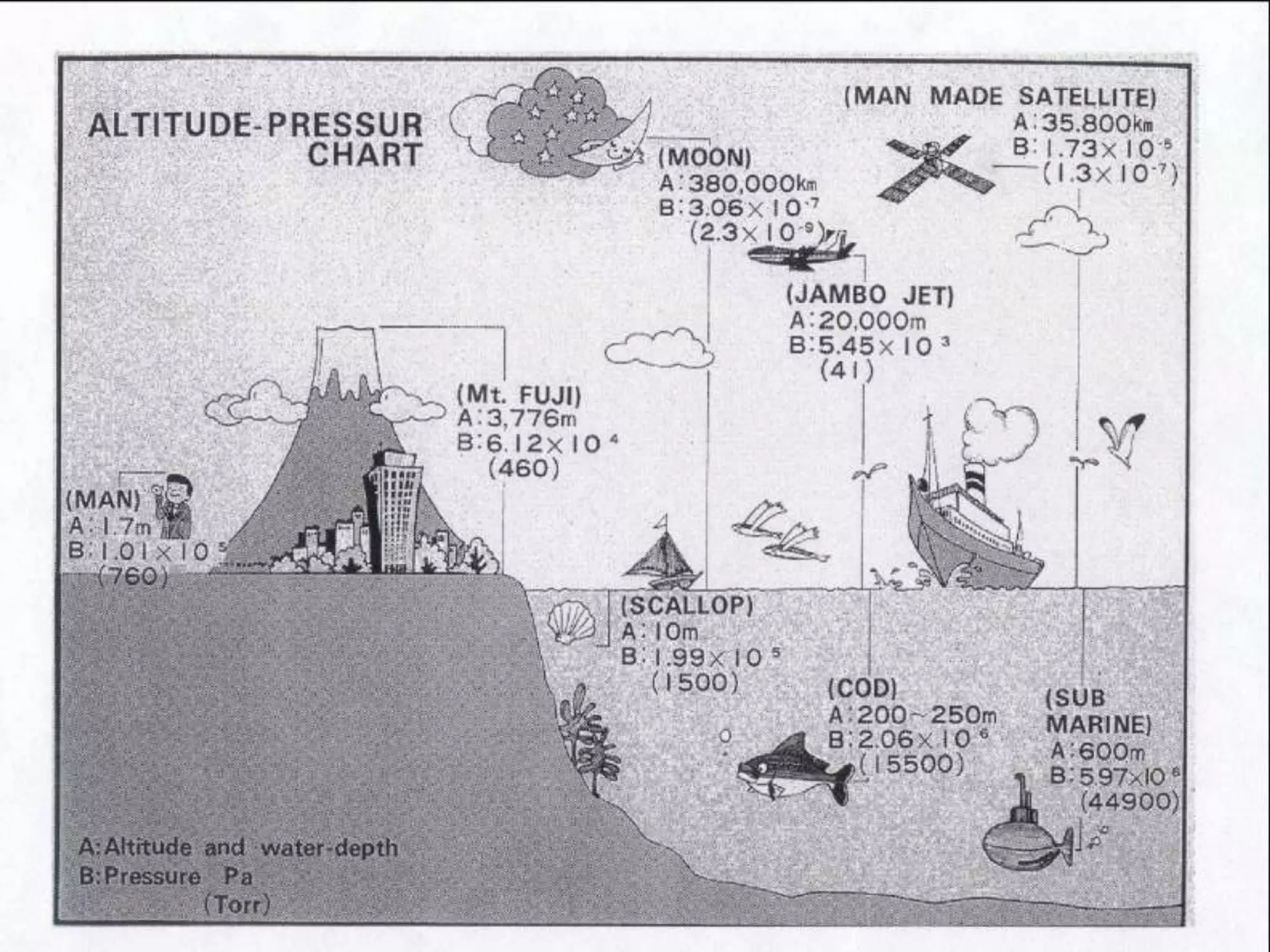
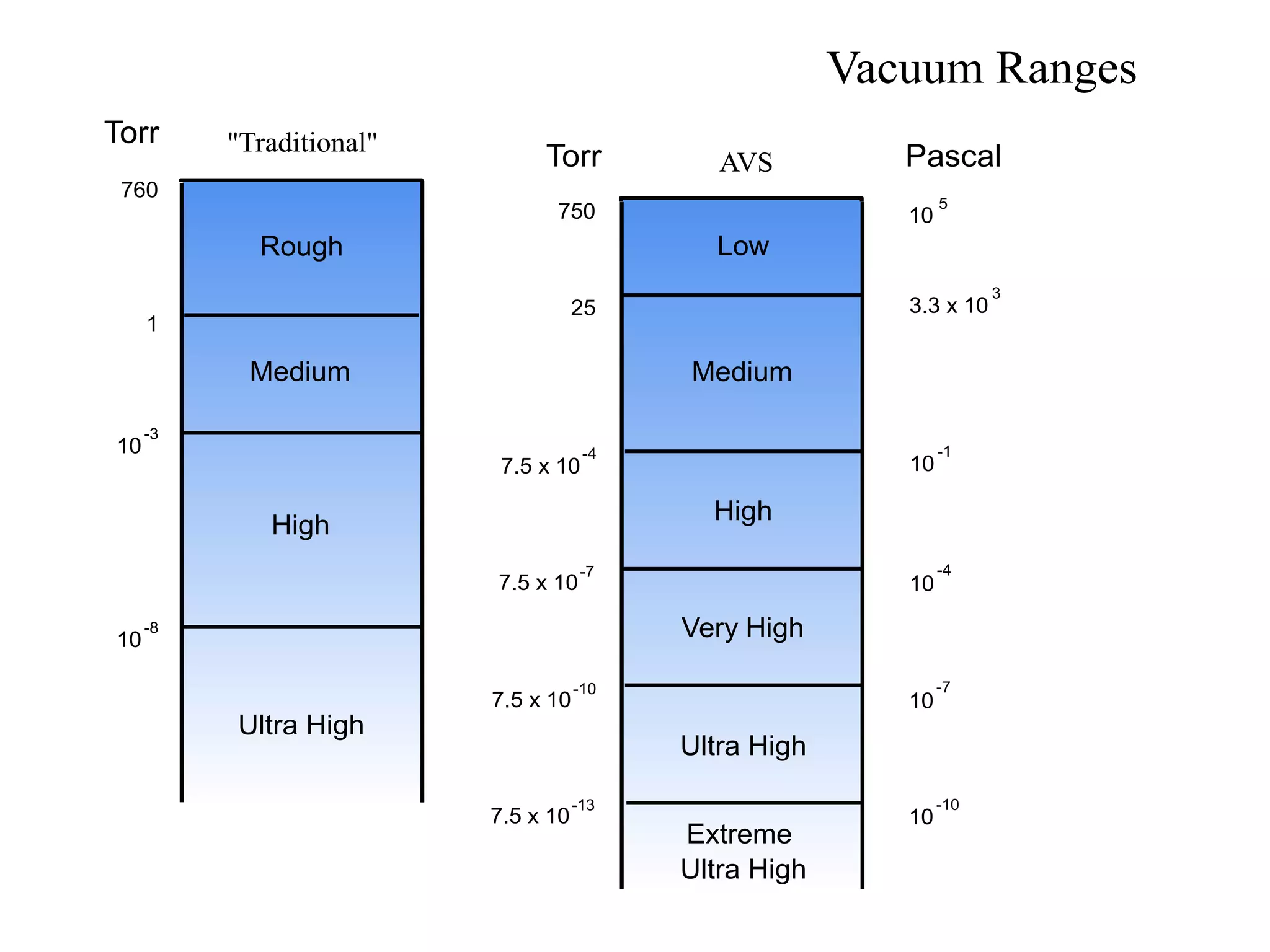
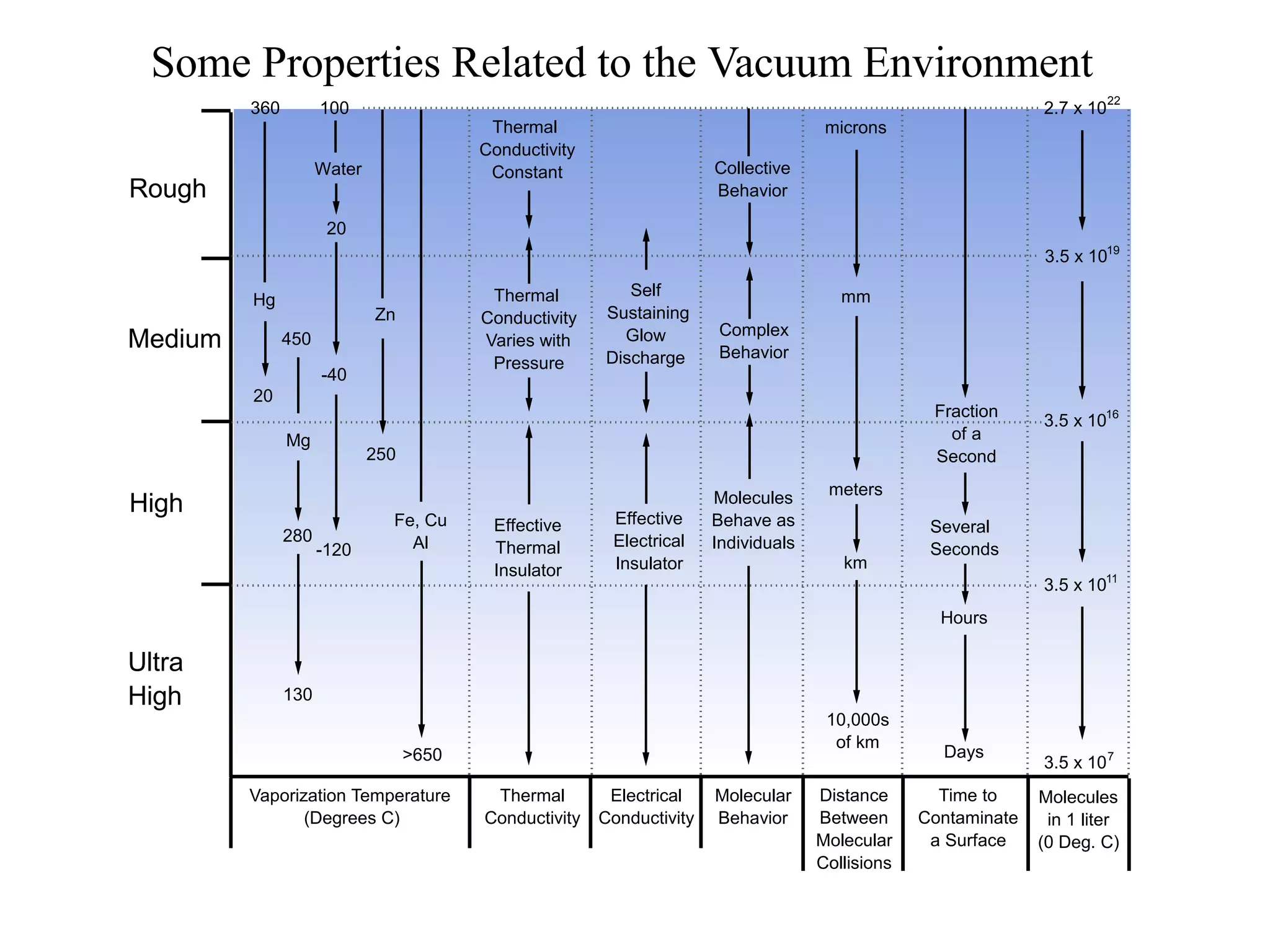
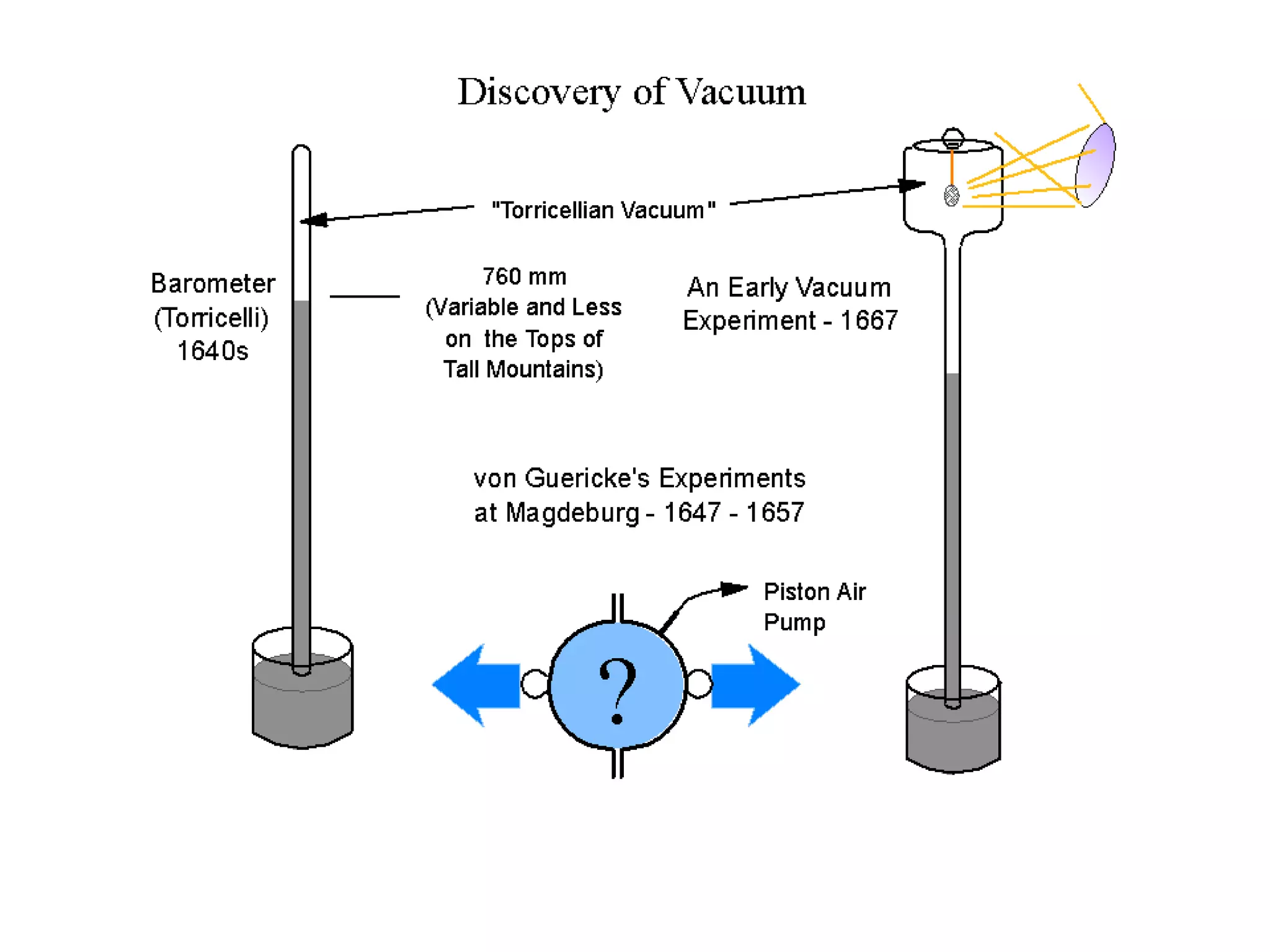
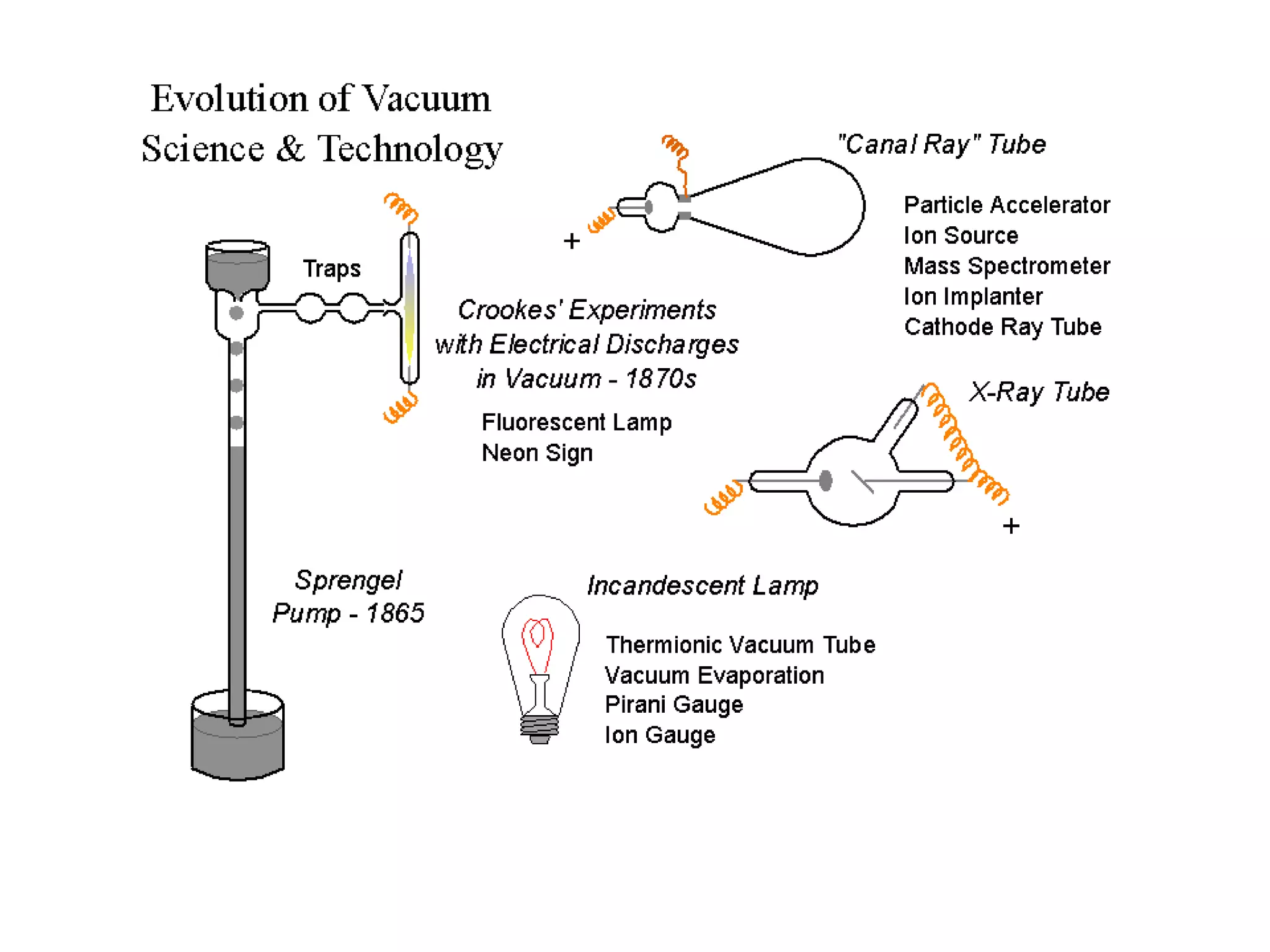
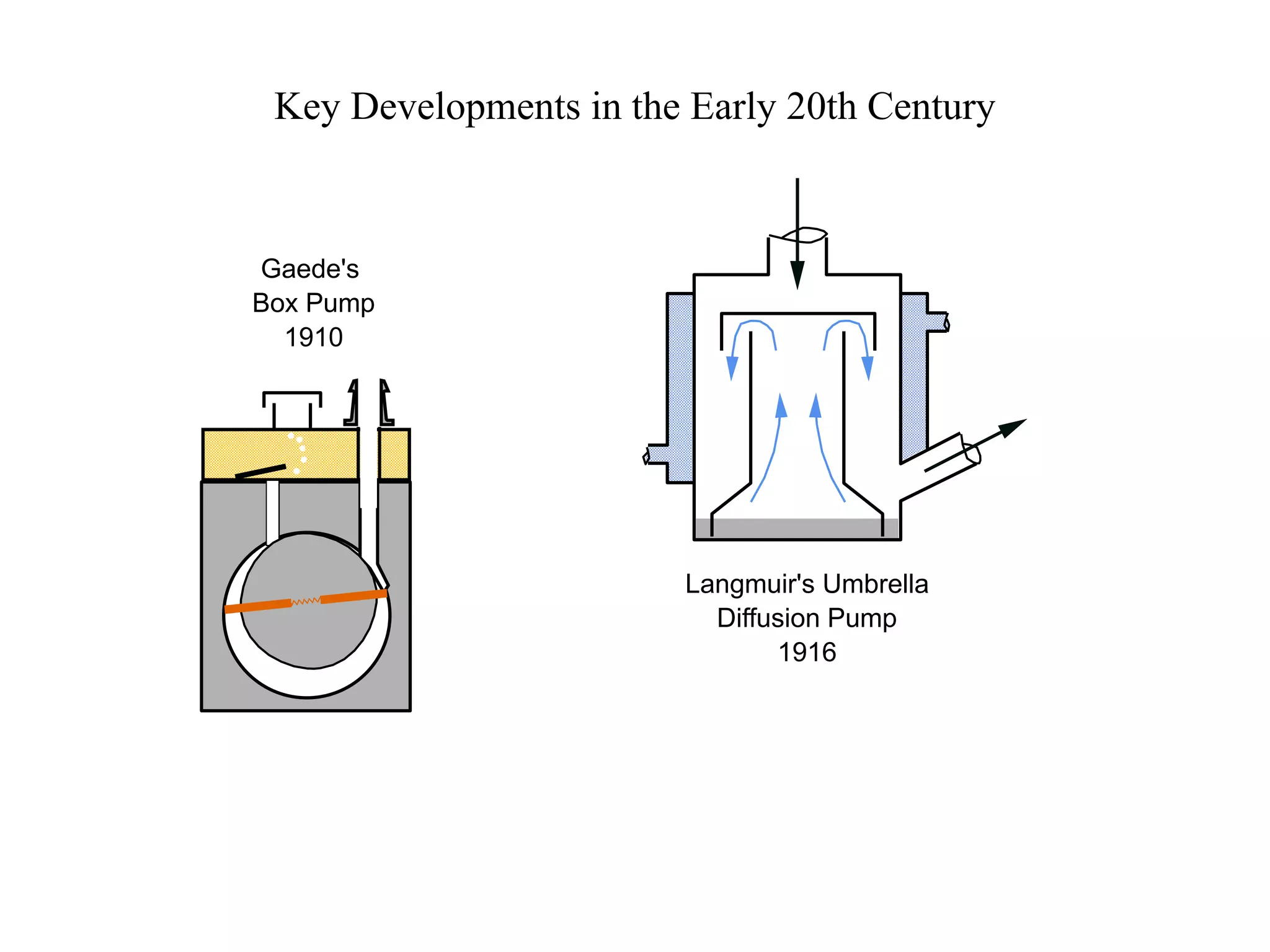
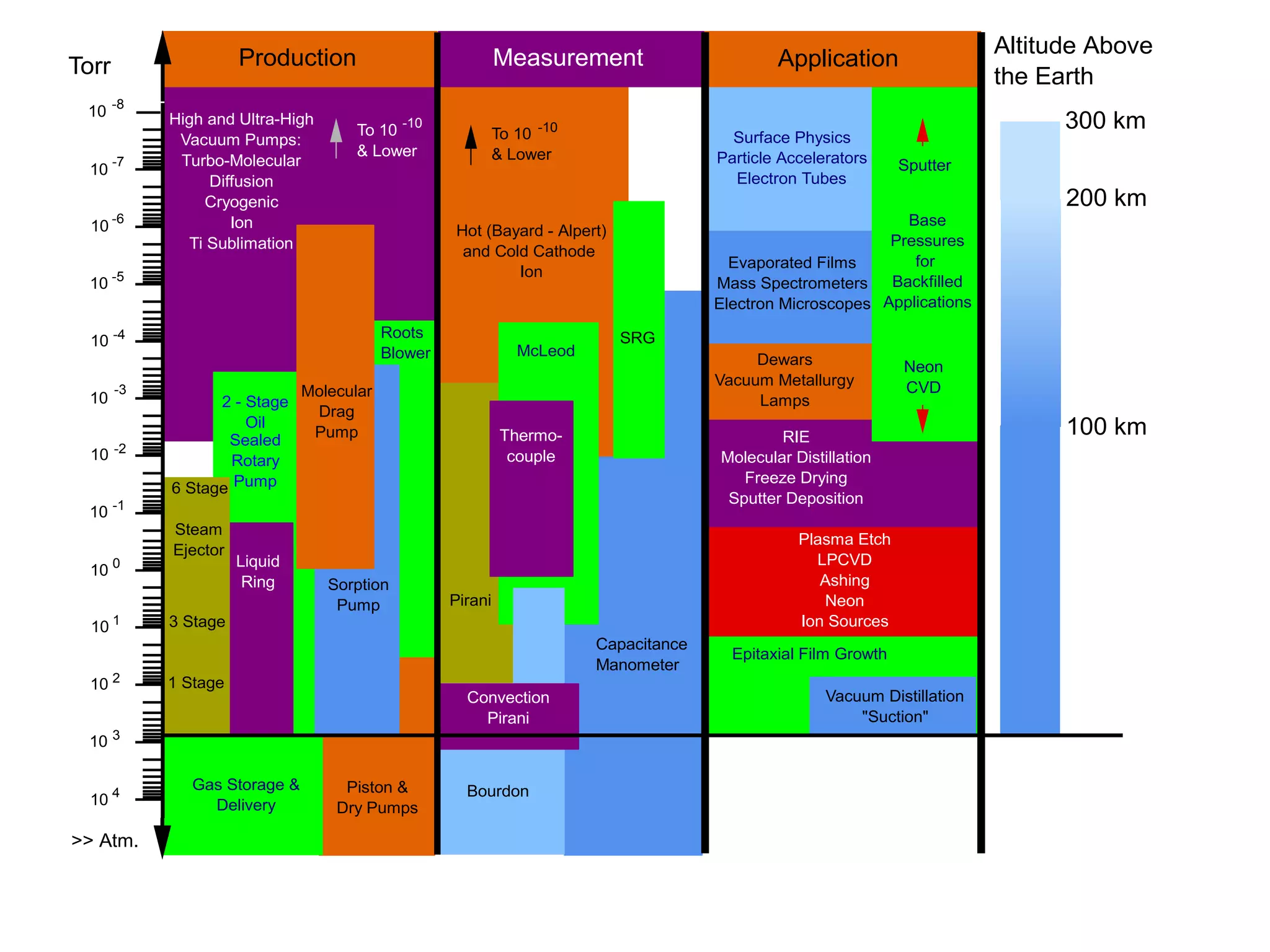
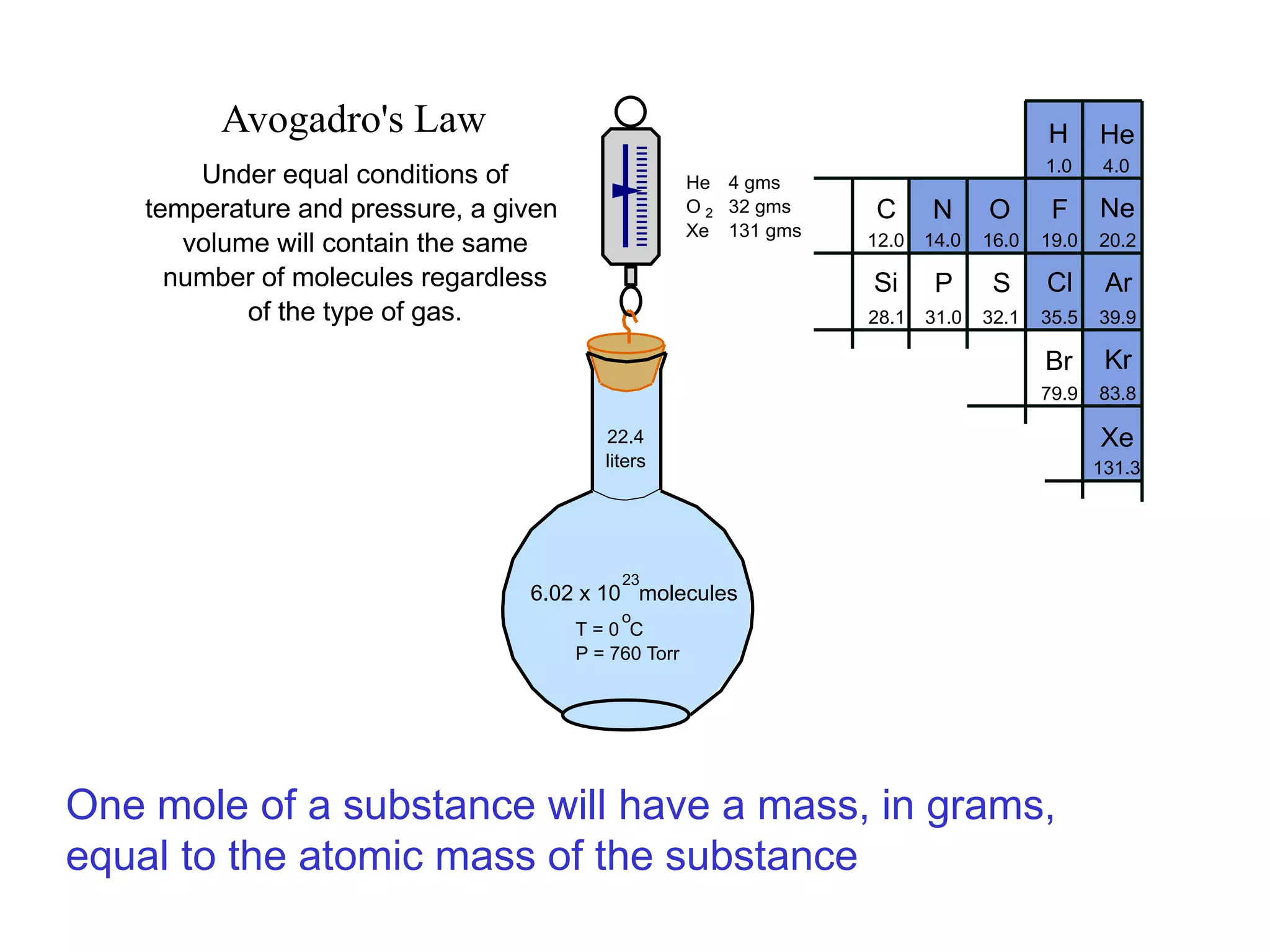
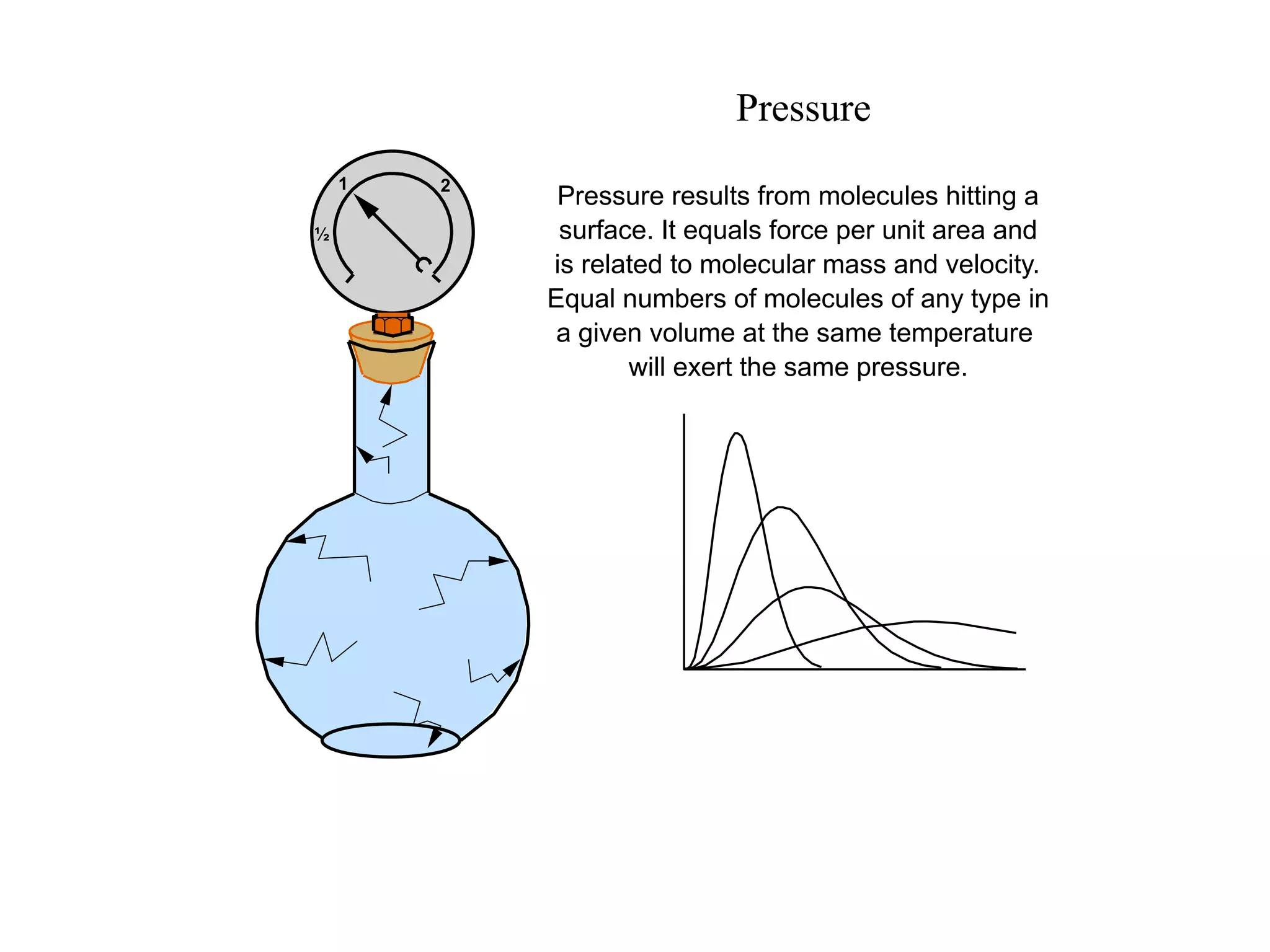
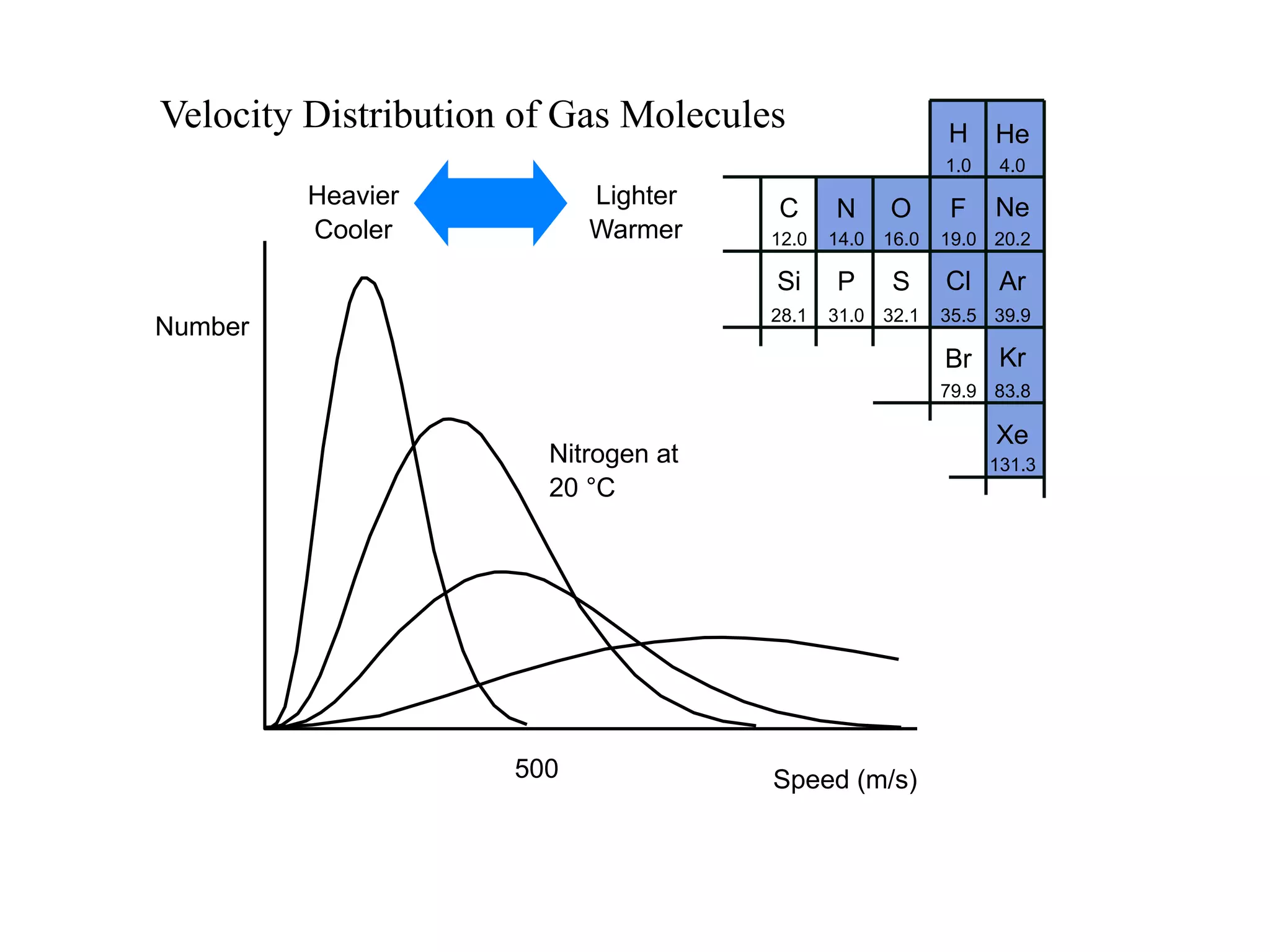
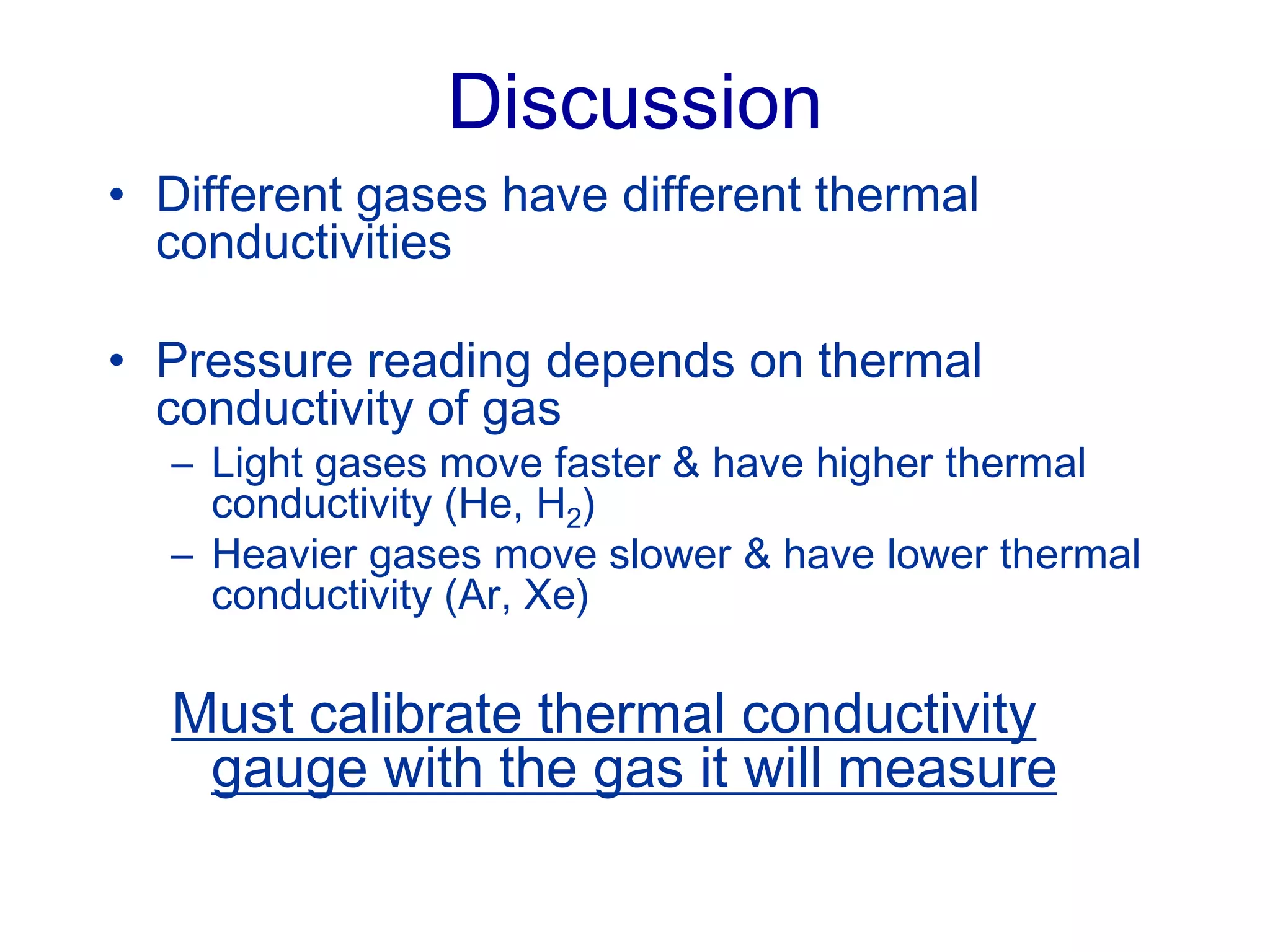
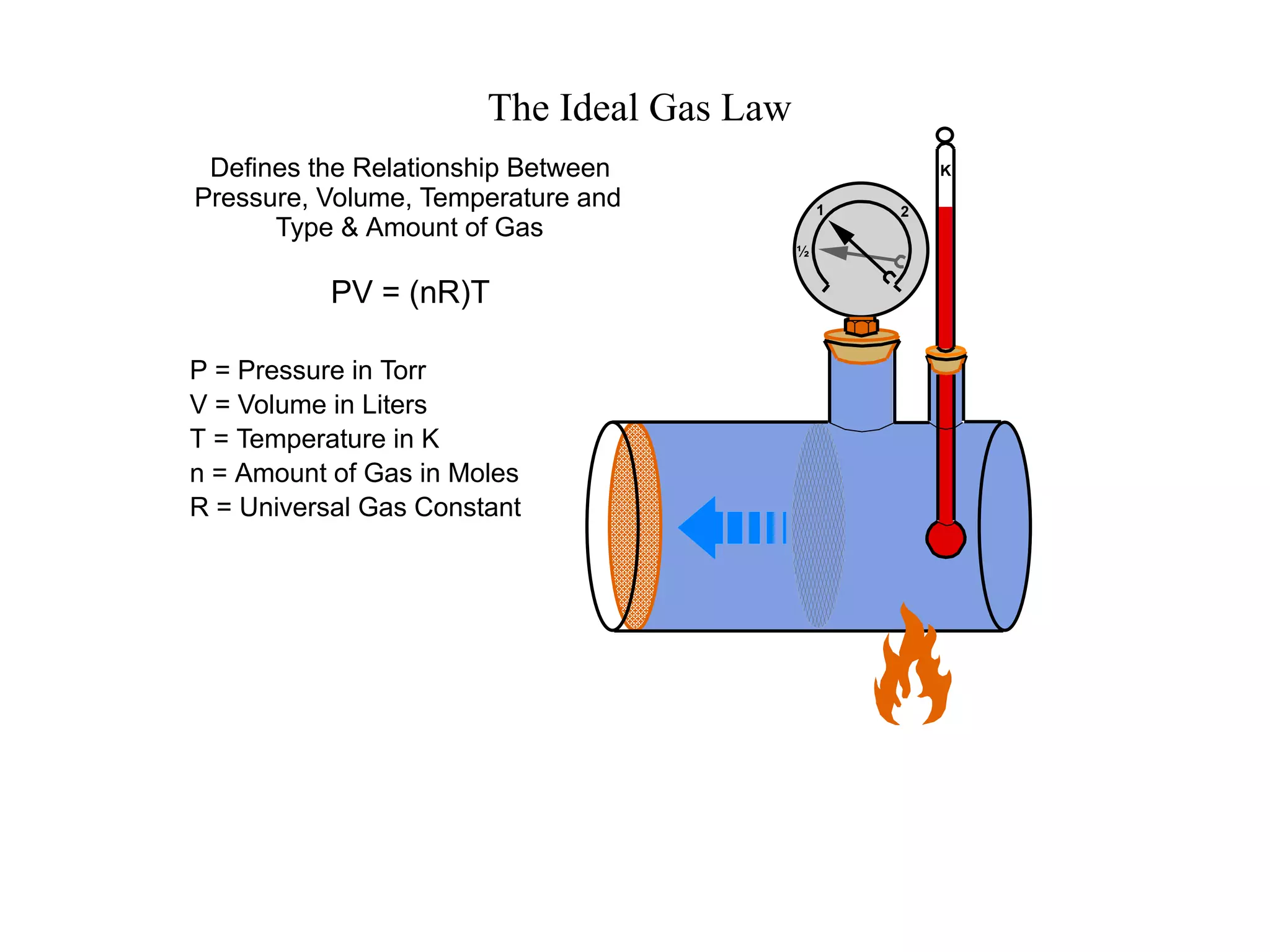
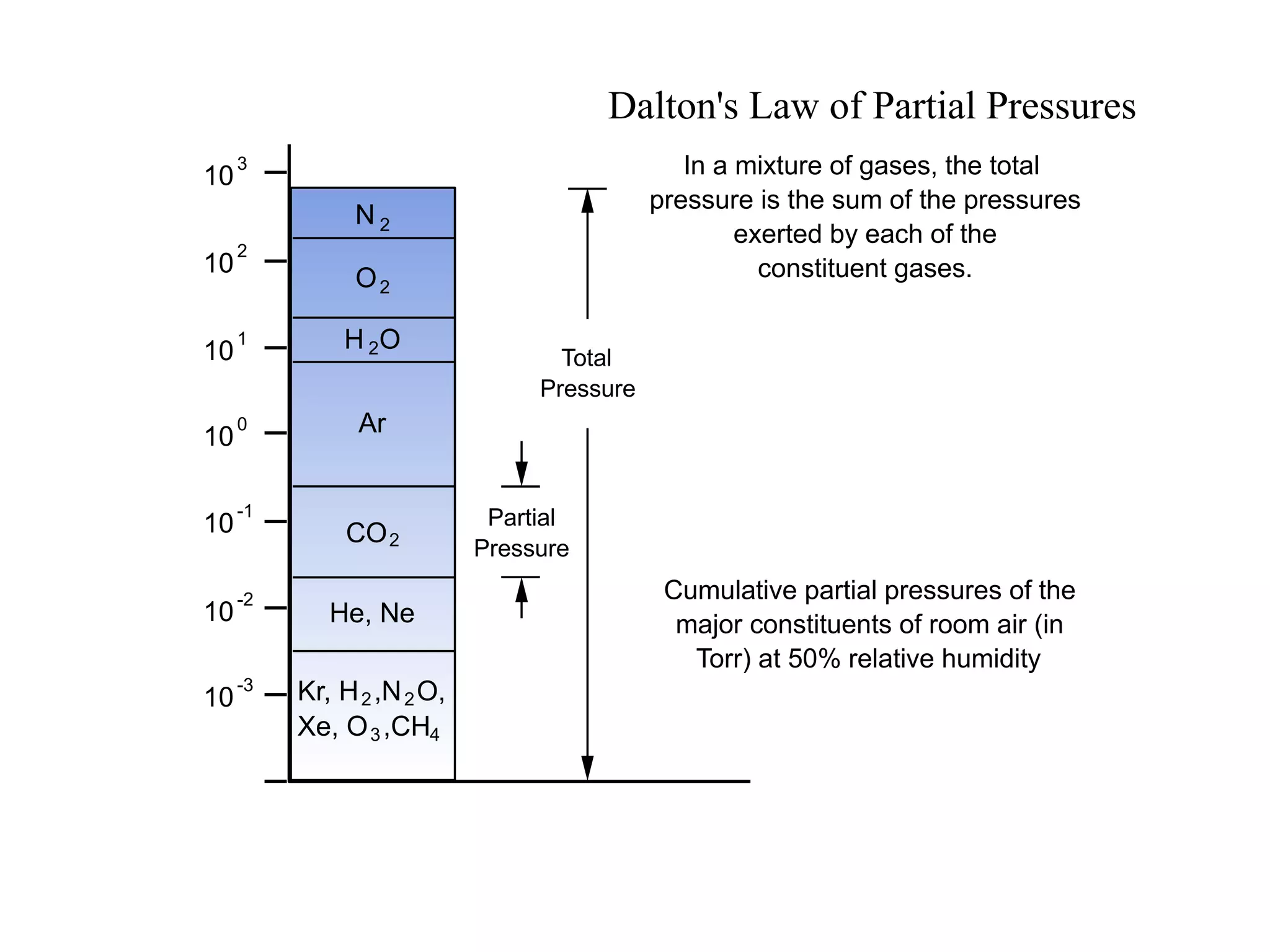
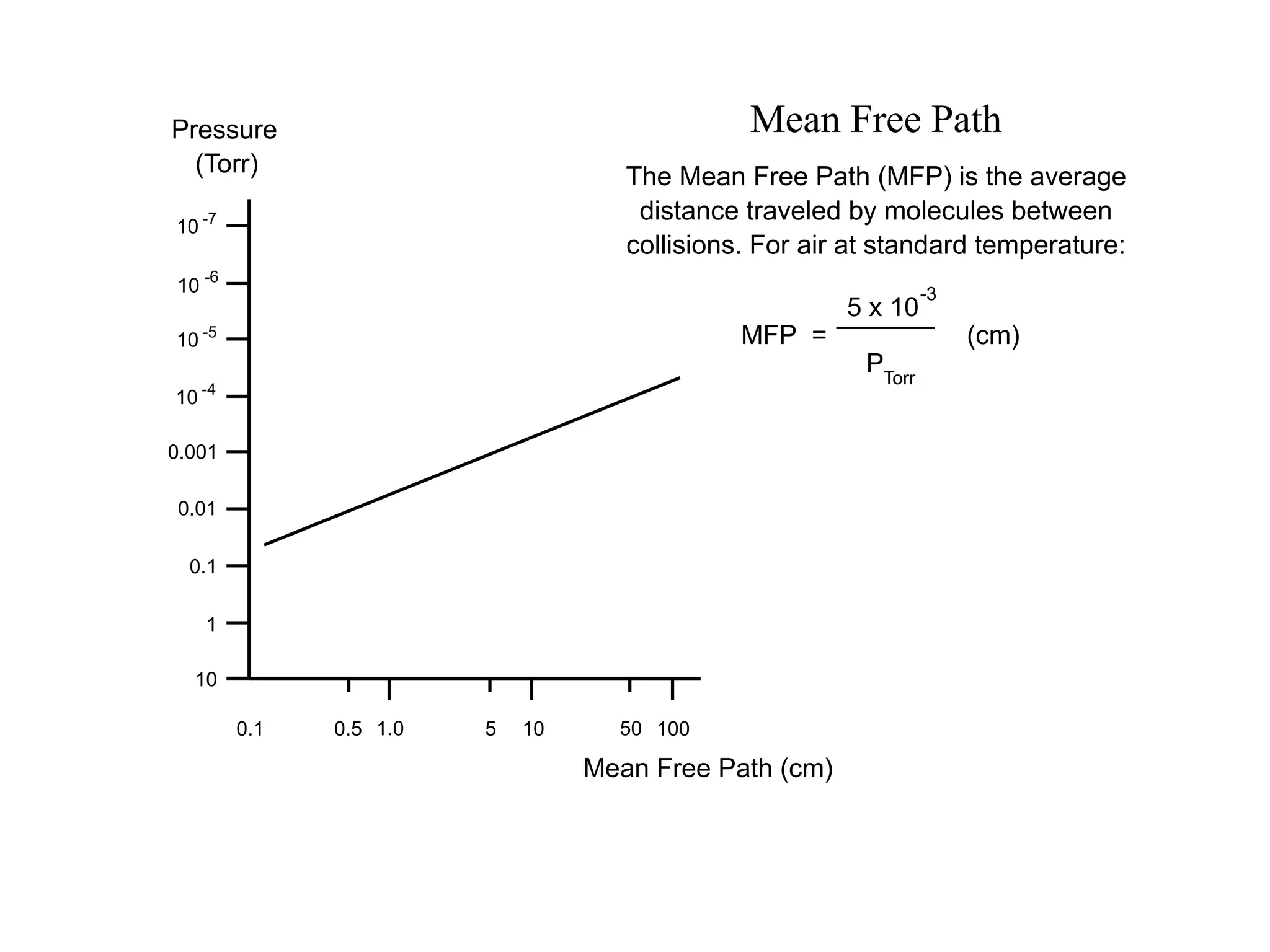
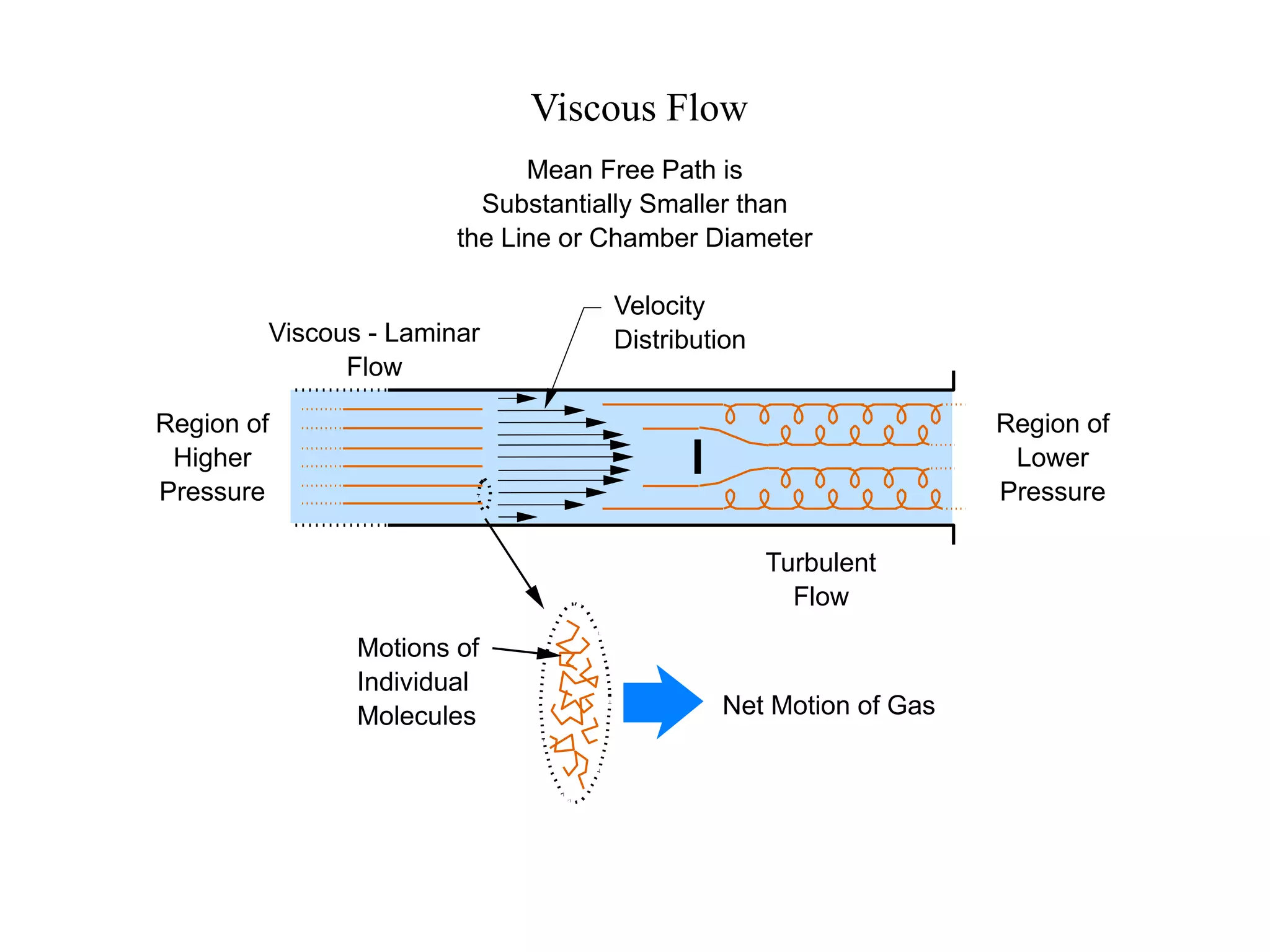
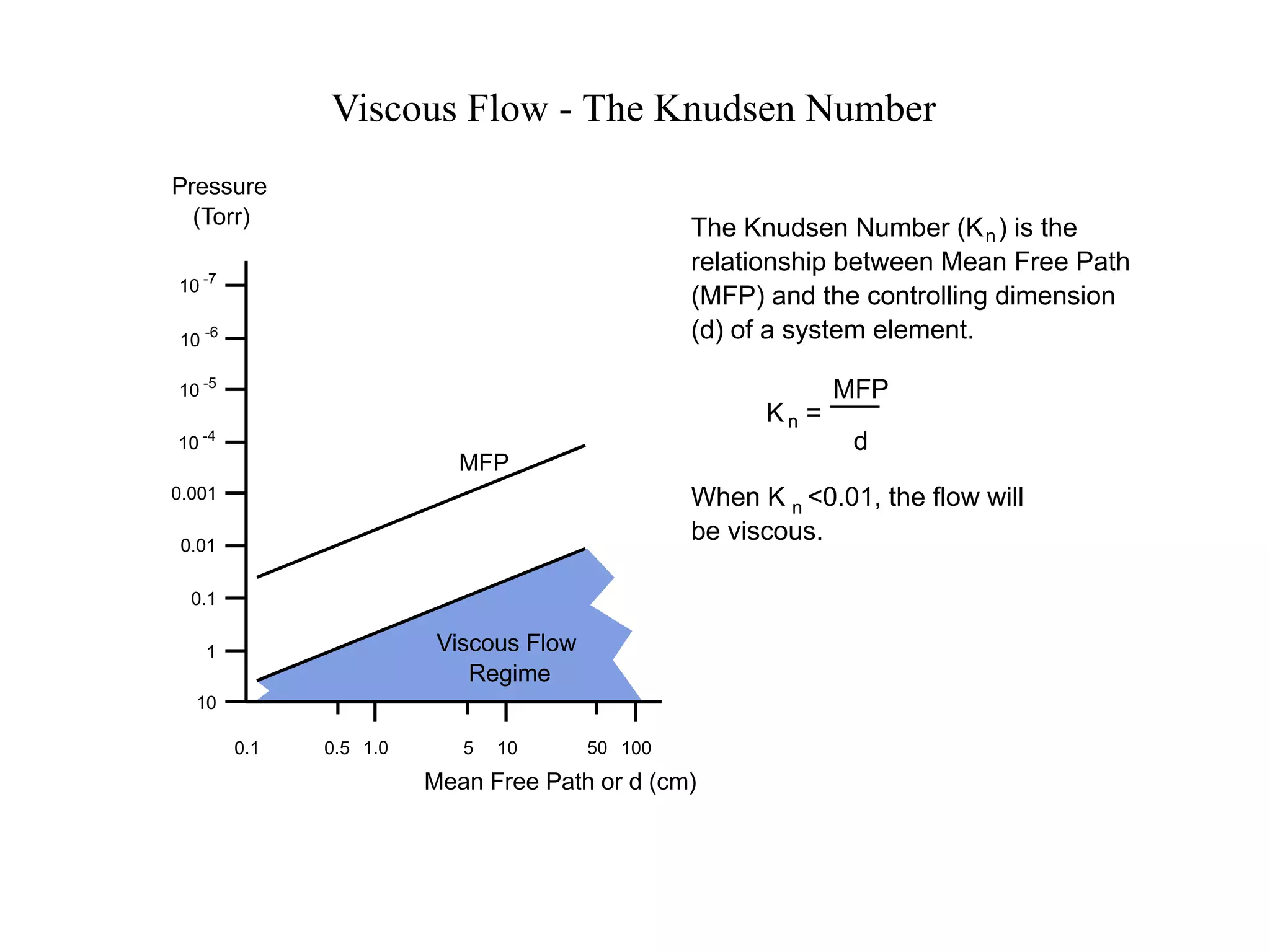
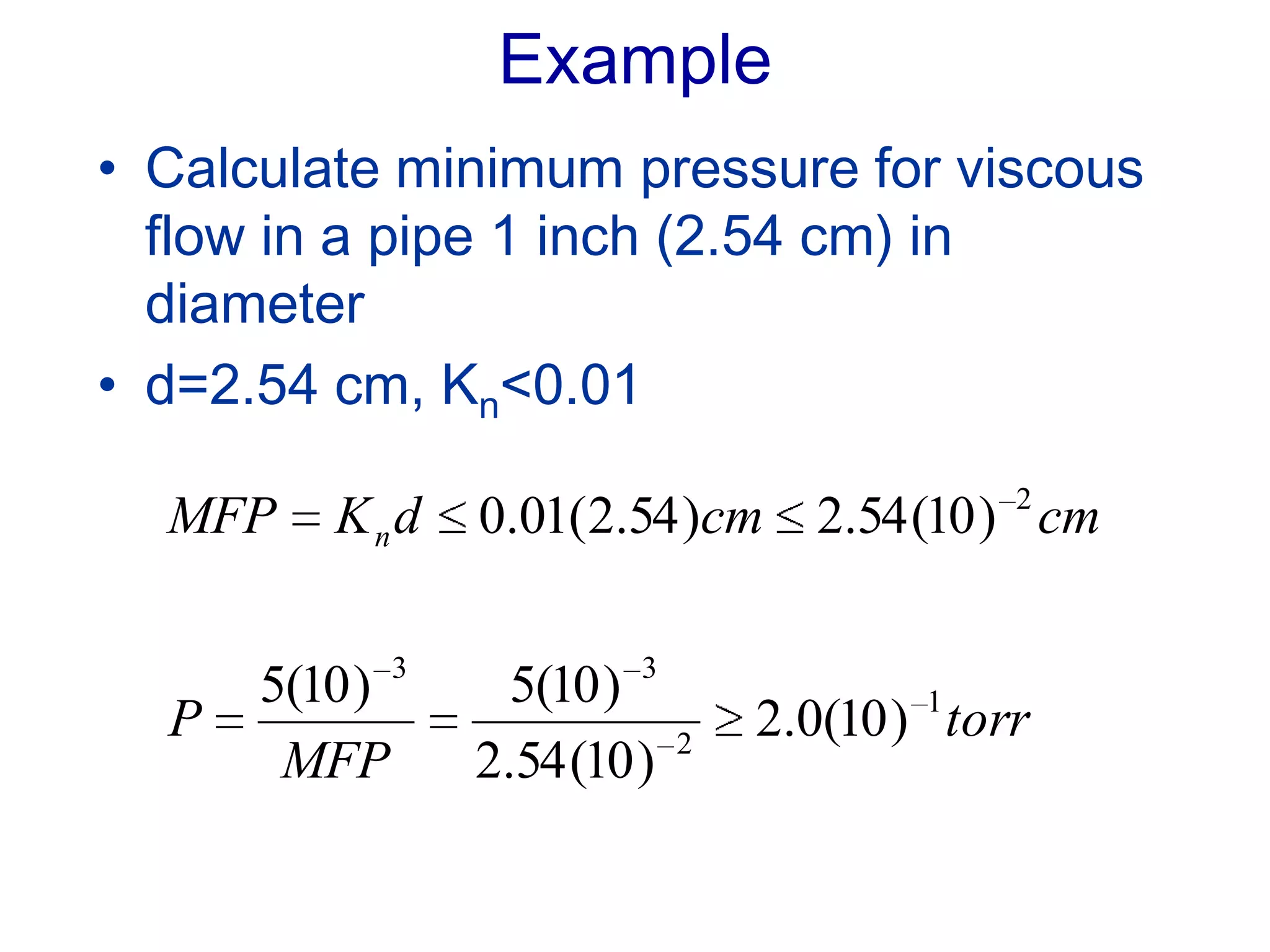
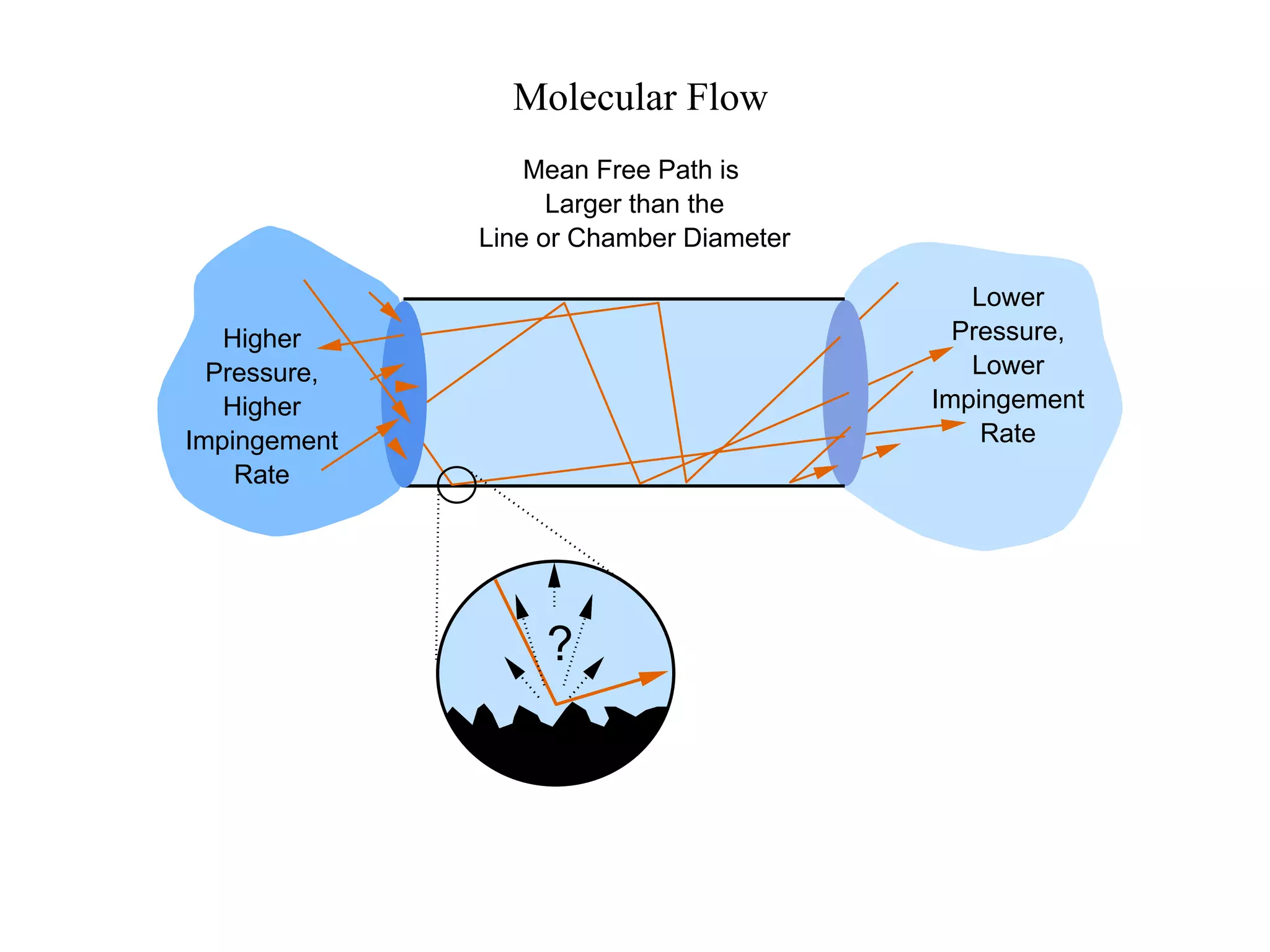
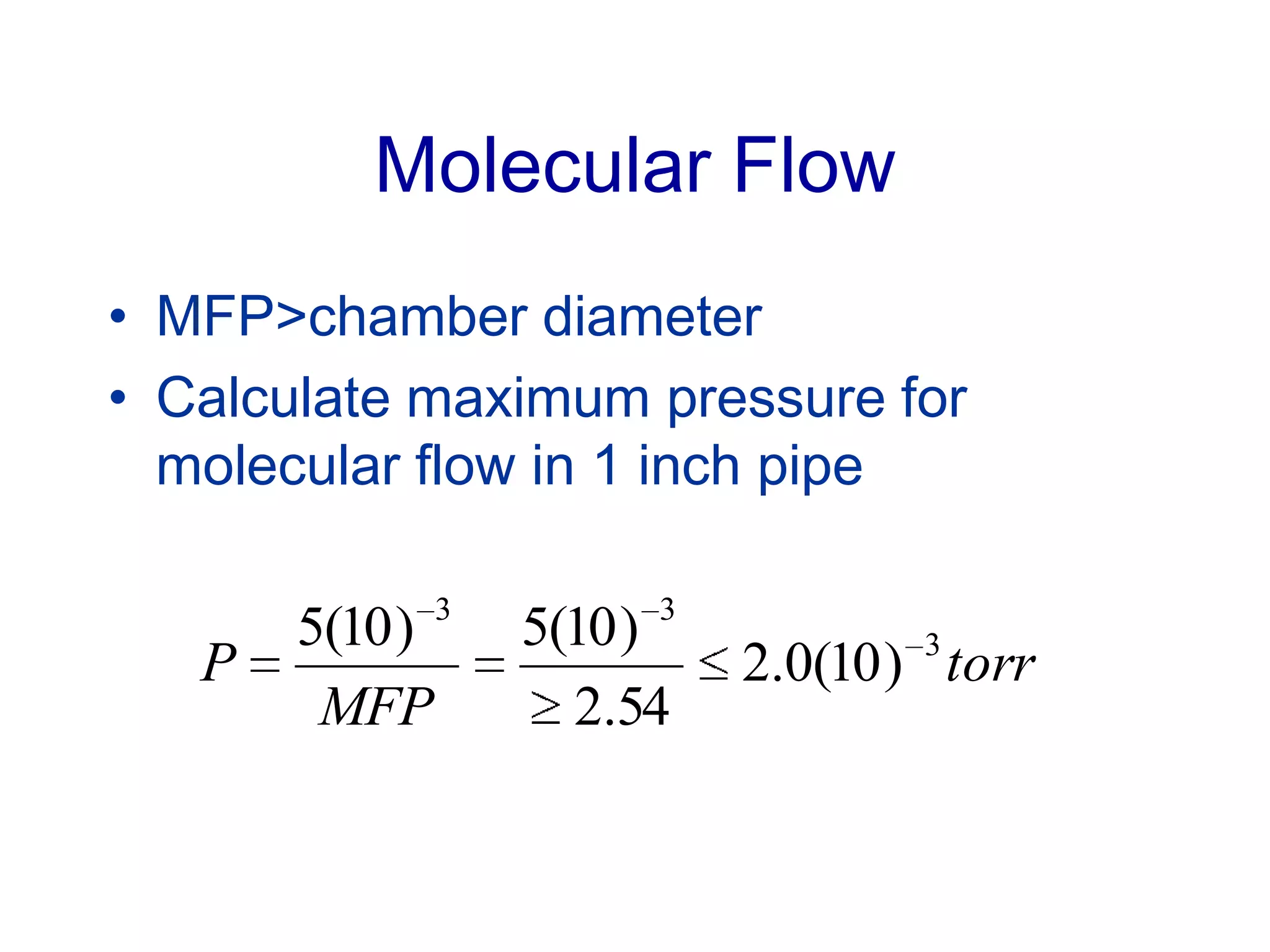
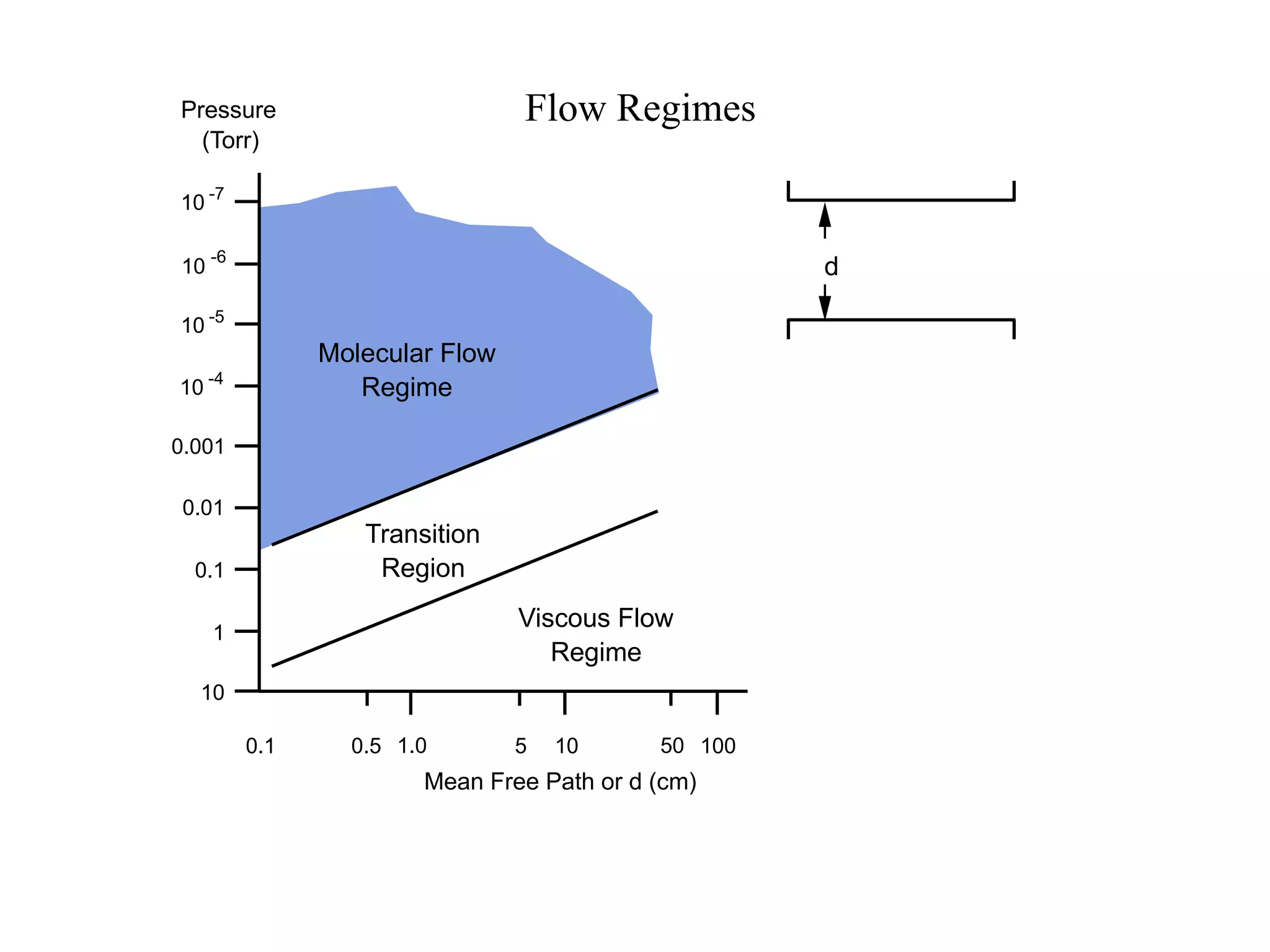
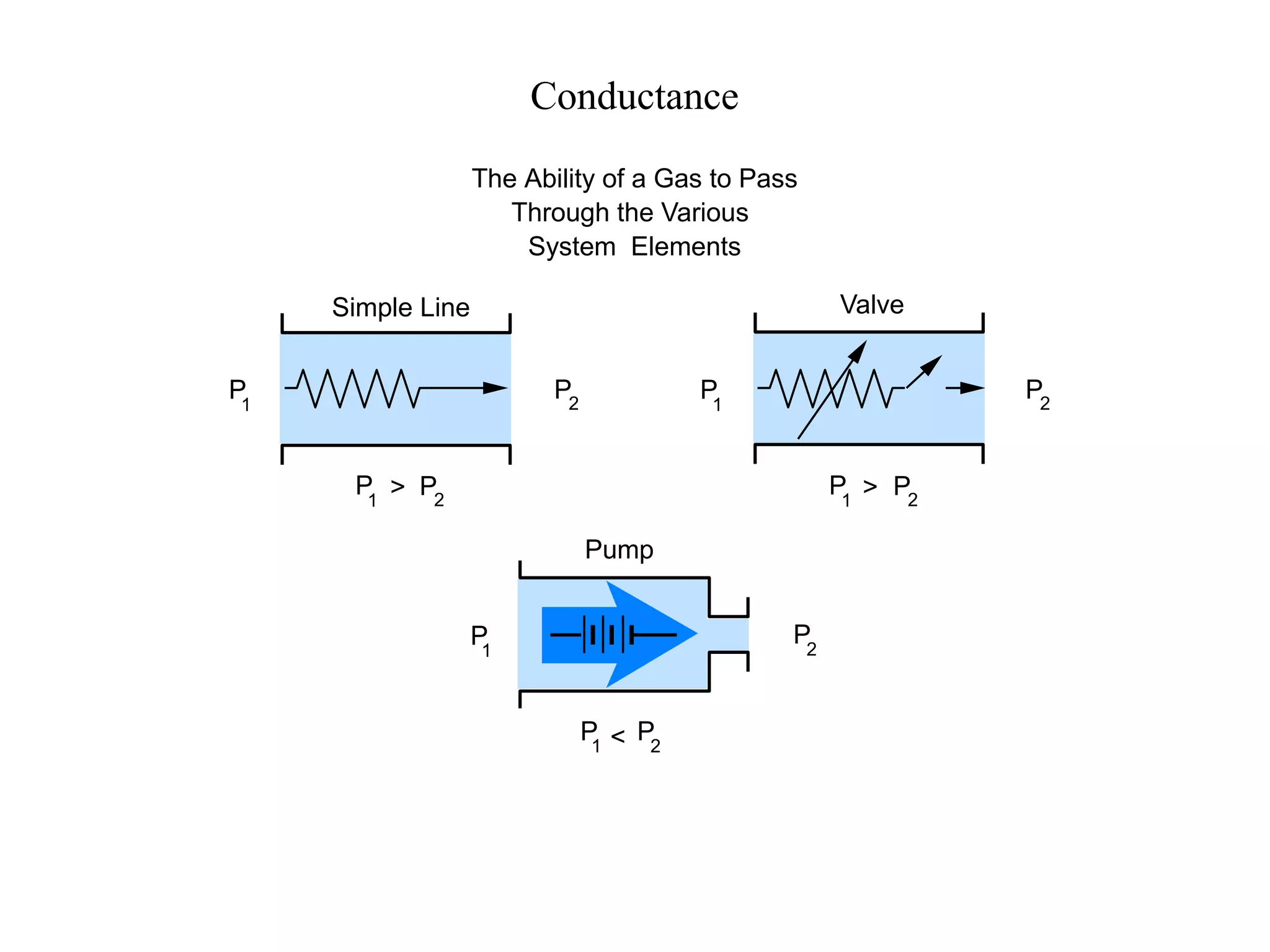
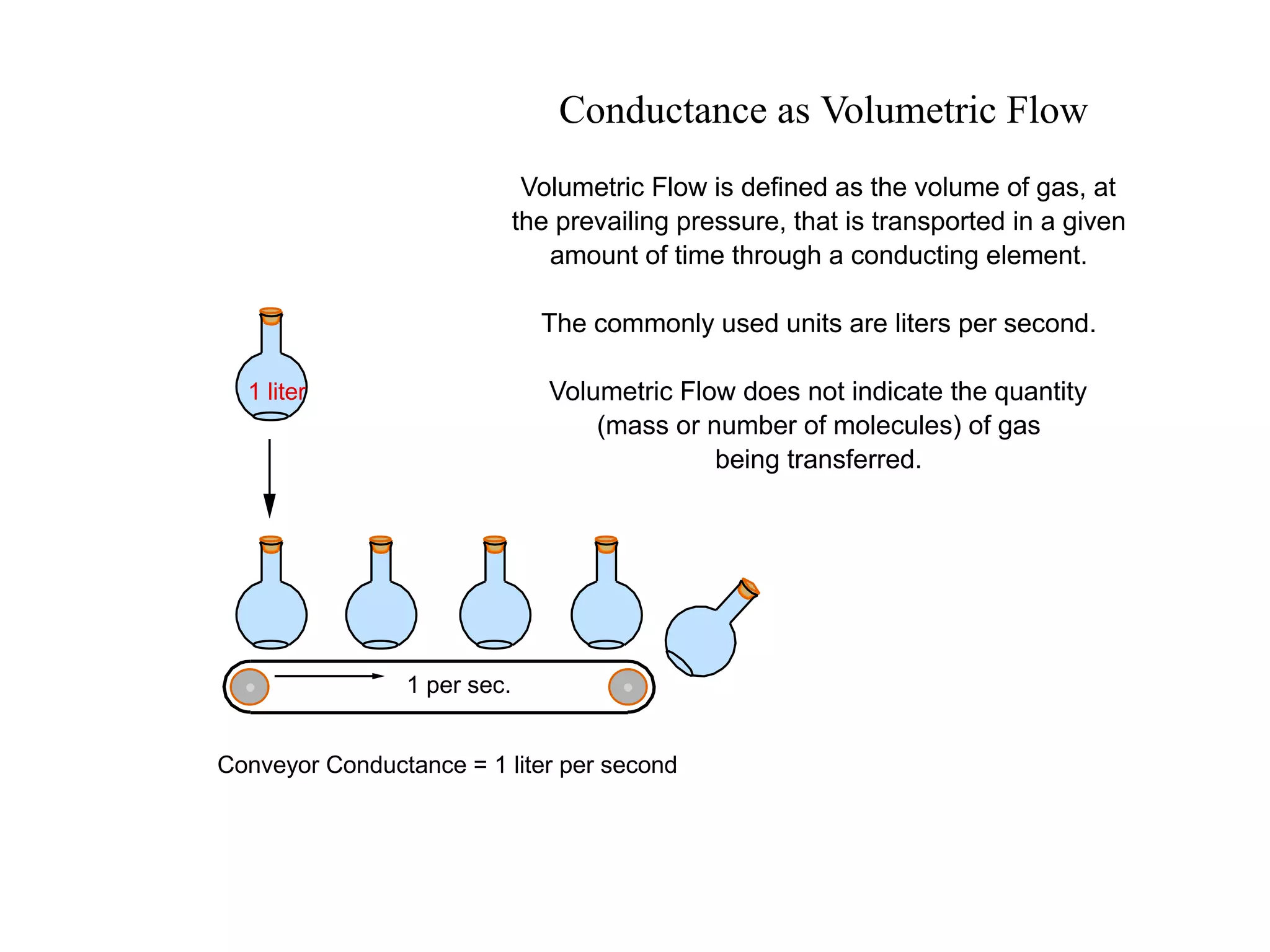
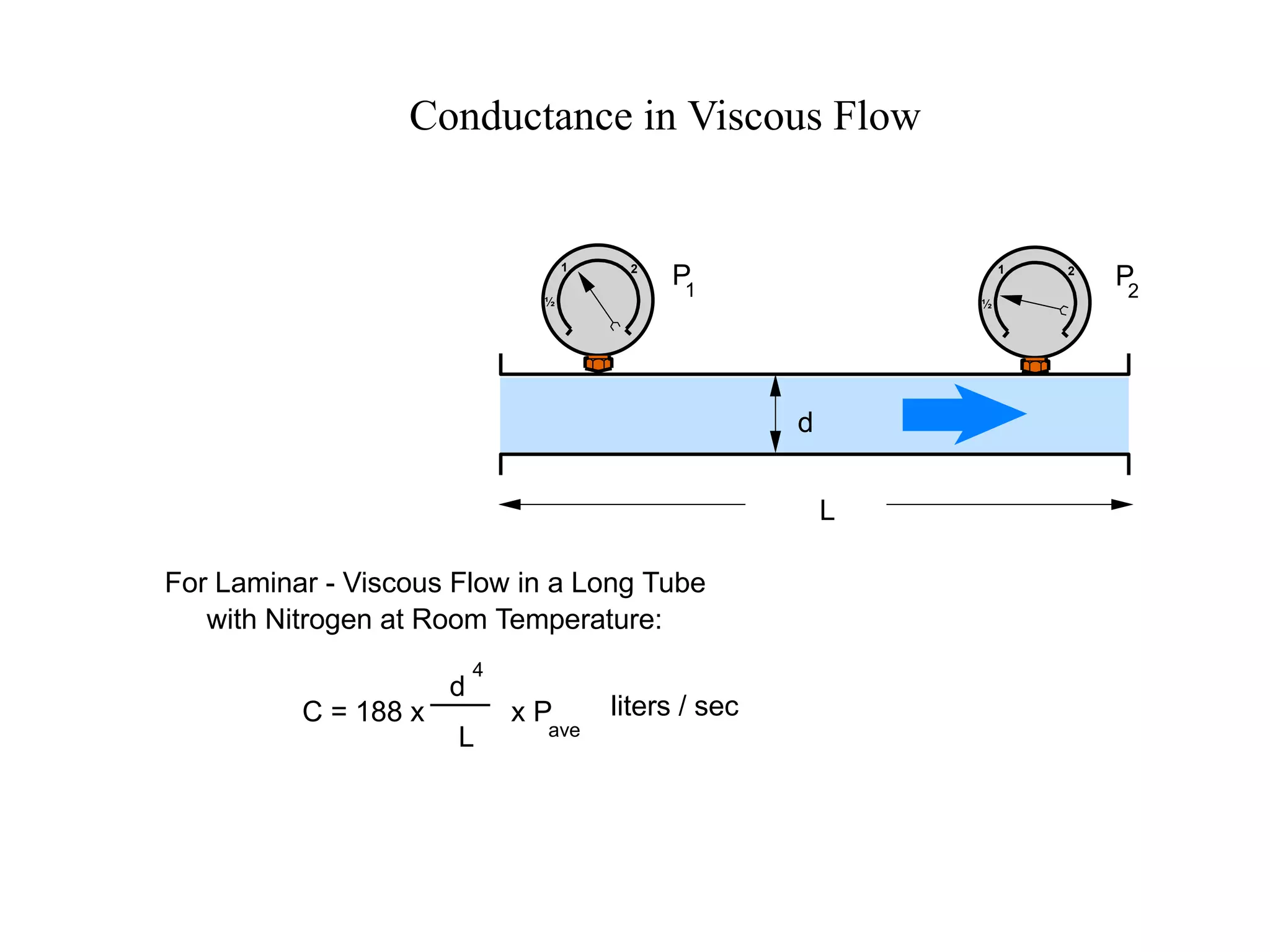
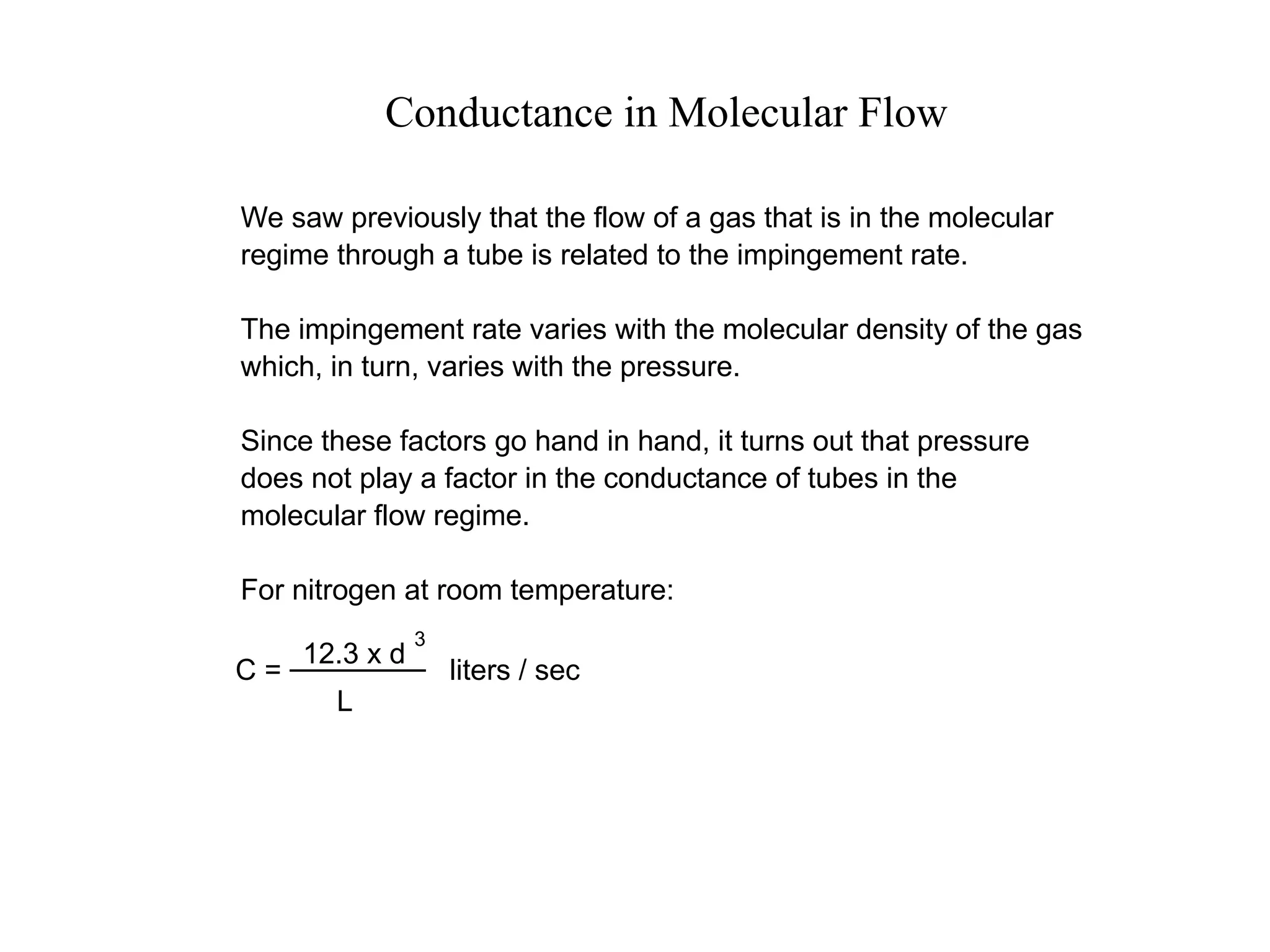
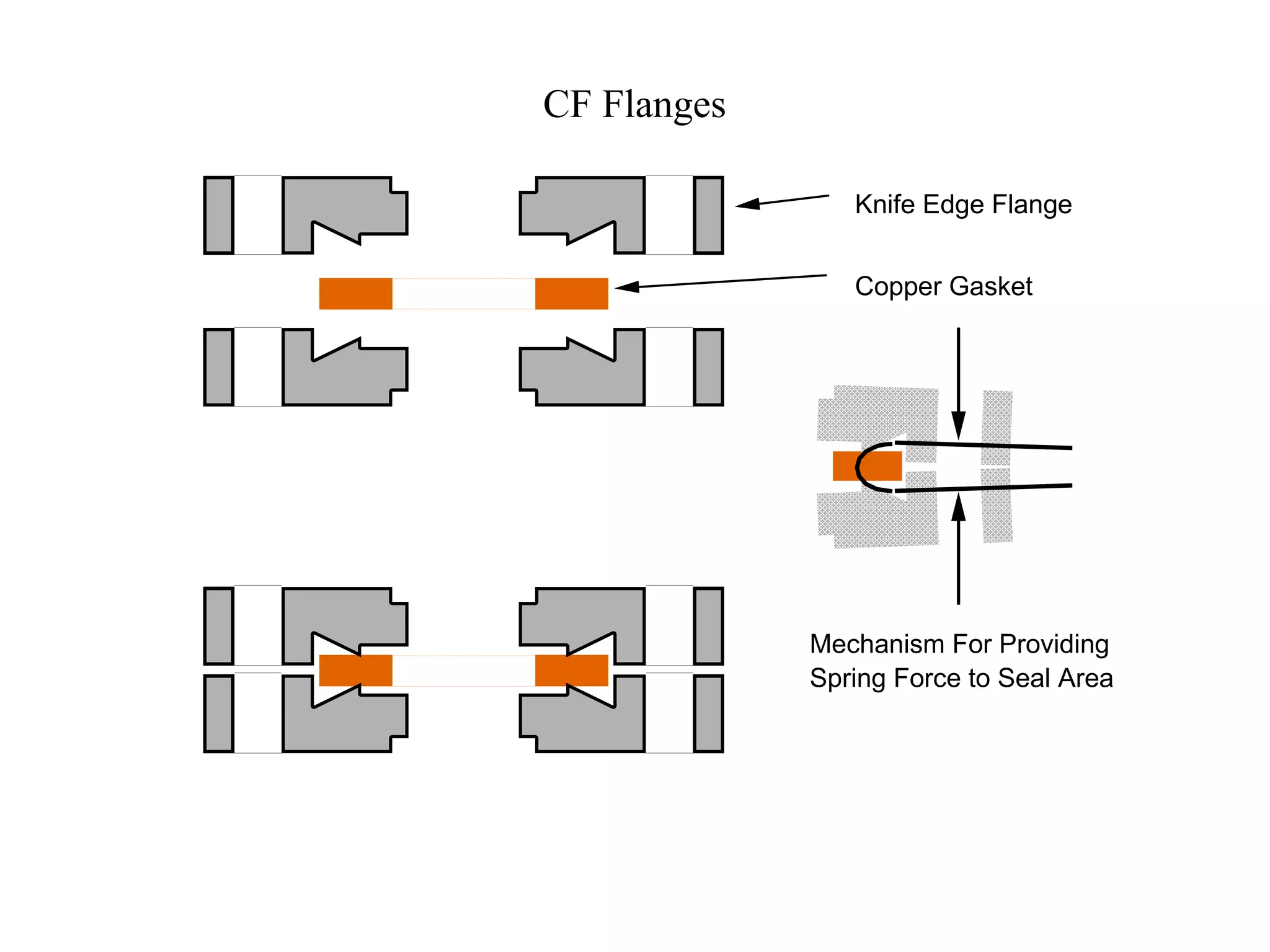
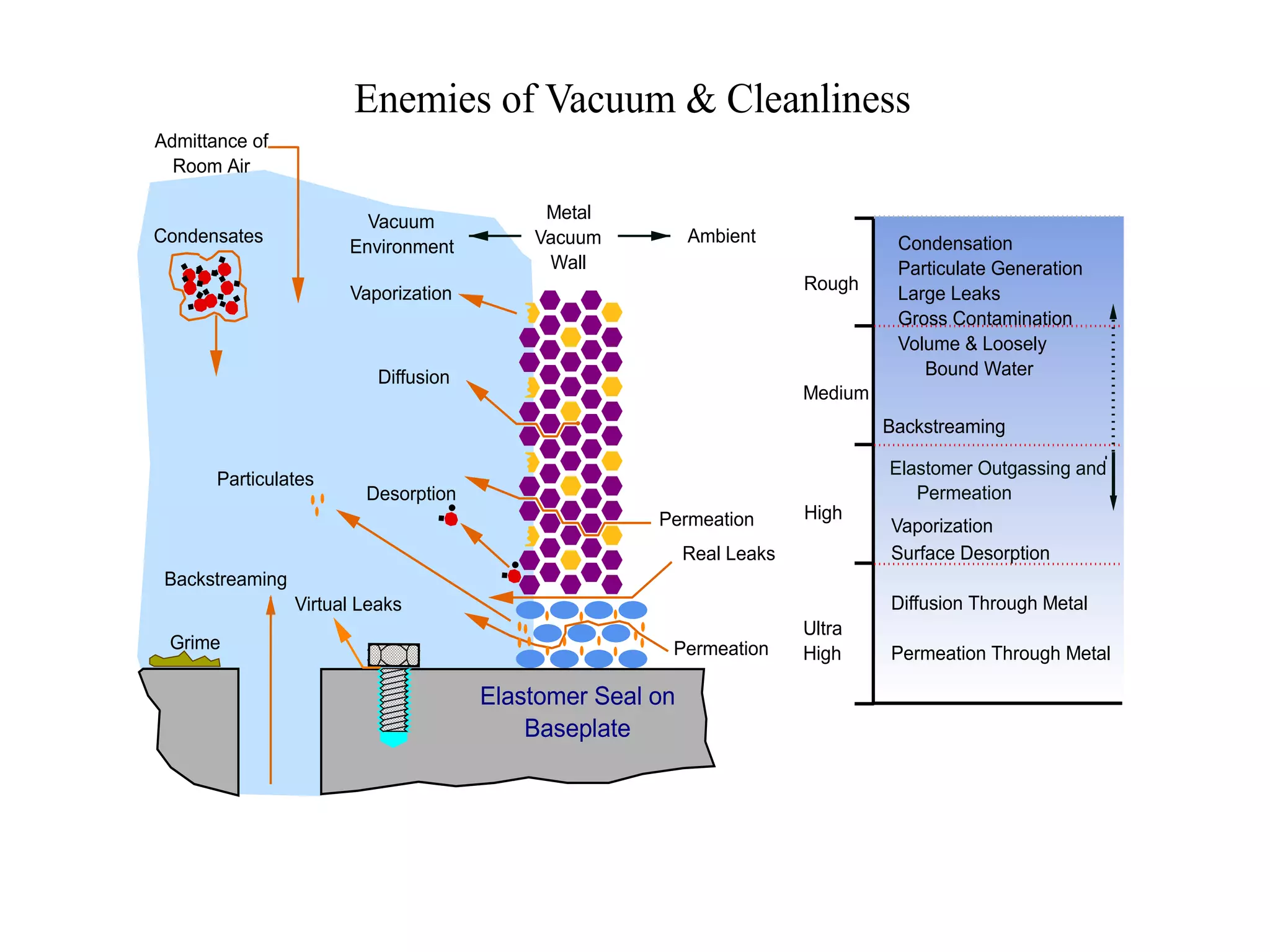
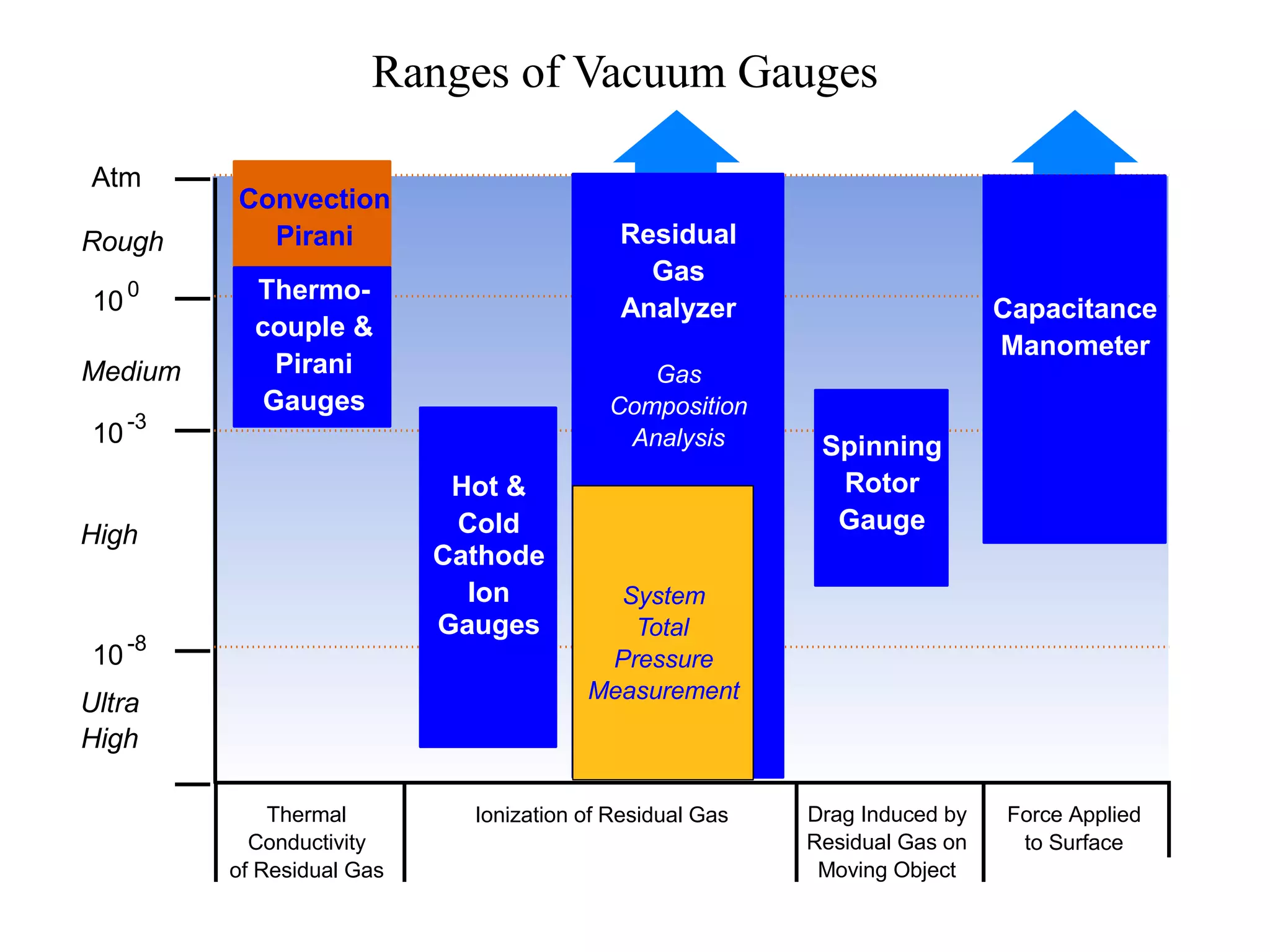
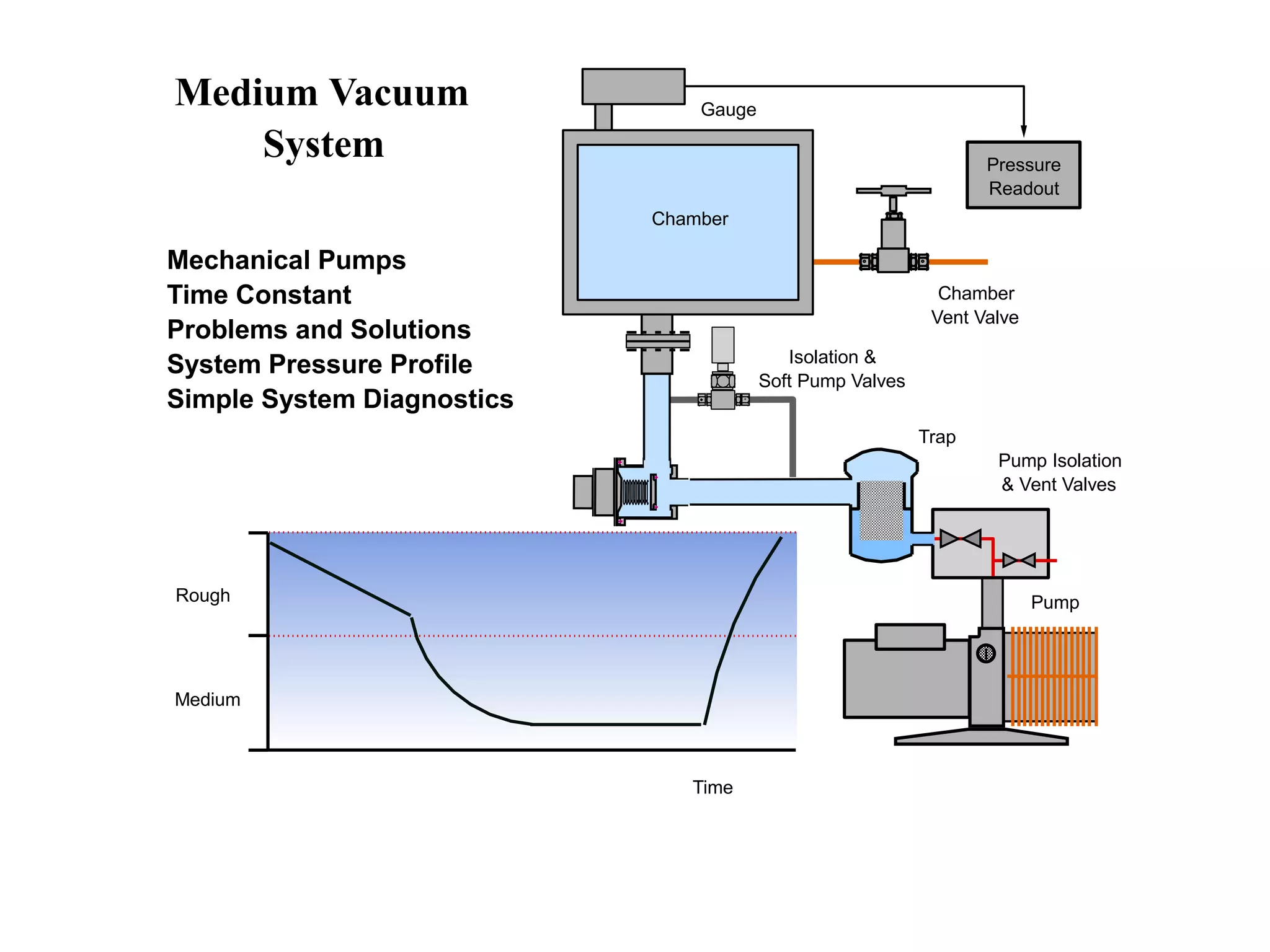
The minimum pressure for viscous flow in a 1 inch diameter pipe is approximately 5 Torr.
At 5 Torr, the mean free path is 0.2 cm.
The Knudsen number is then:
Kn = MFP/d
= 0.2 cm / 2.54 cm
= 0.078
Since Kn < 0.01, the flow will be viscous at or below 5 Torr pressure.