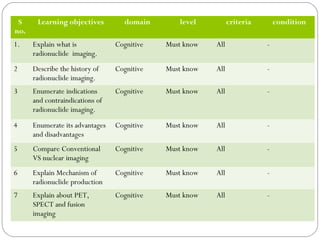
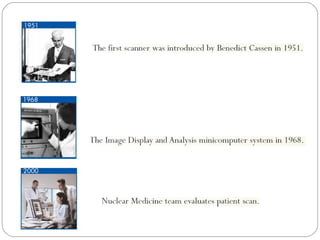
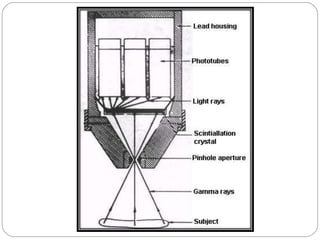
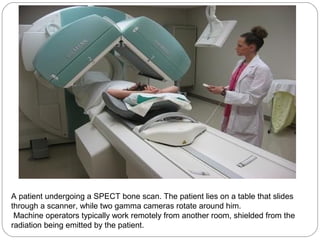
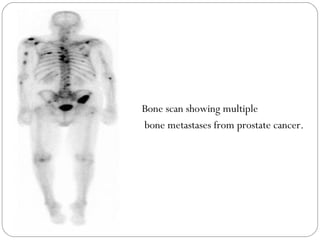
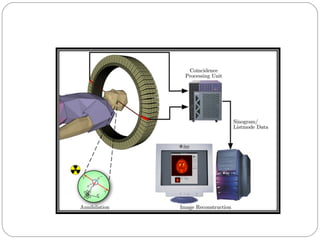
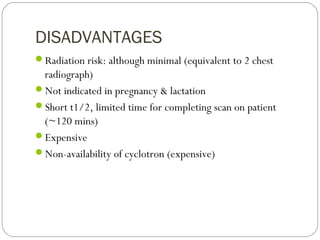
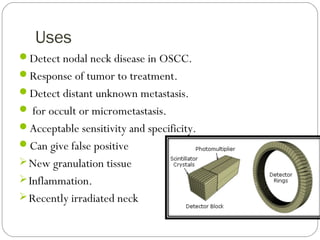
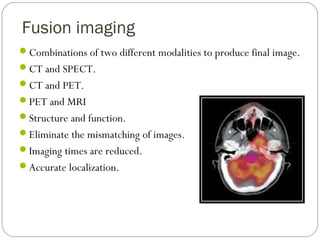
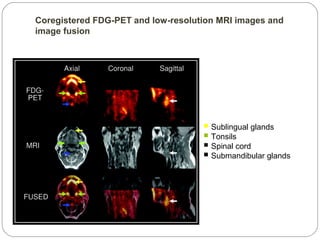
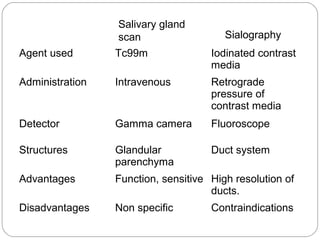
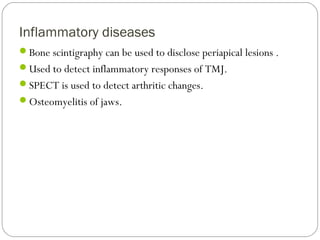
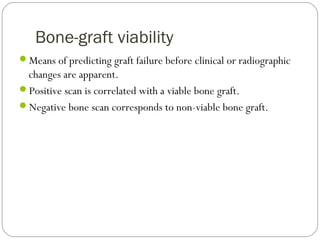
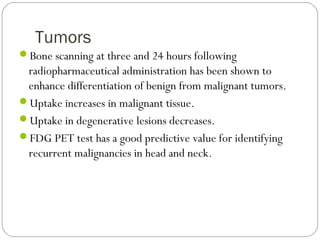
This document provides an overview of a seminar presentation on radionuclide imaging. The presentation aims to explain radionuclide imaging, its history, indications, contraindications, advantages, disadvantages, and newer techniques like SPECT, PET, and PET-CT. It discusses the basics of radionuclide production and imaging, including the mechanisms, equipment, and applications of various nuclear medicine procedures like bone scans, lymphoscintigraphy, and salivary gland imaging.