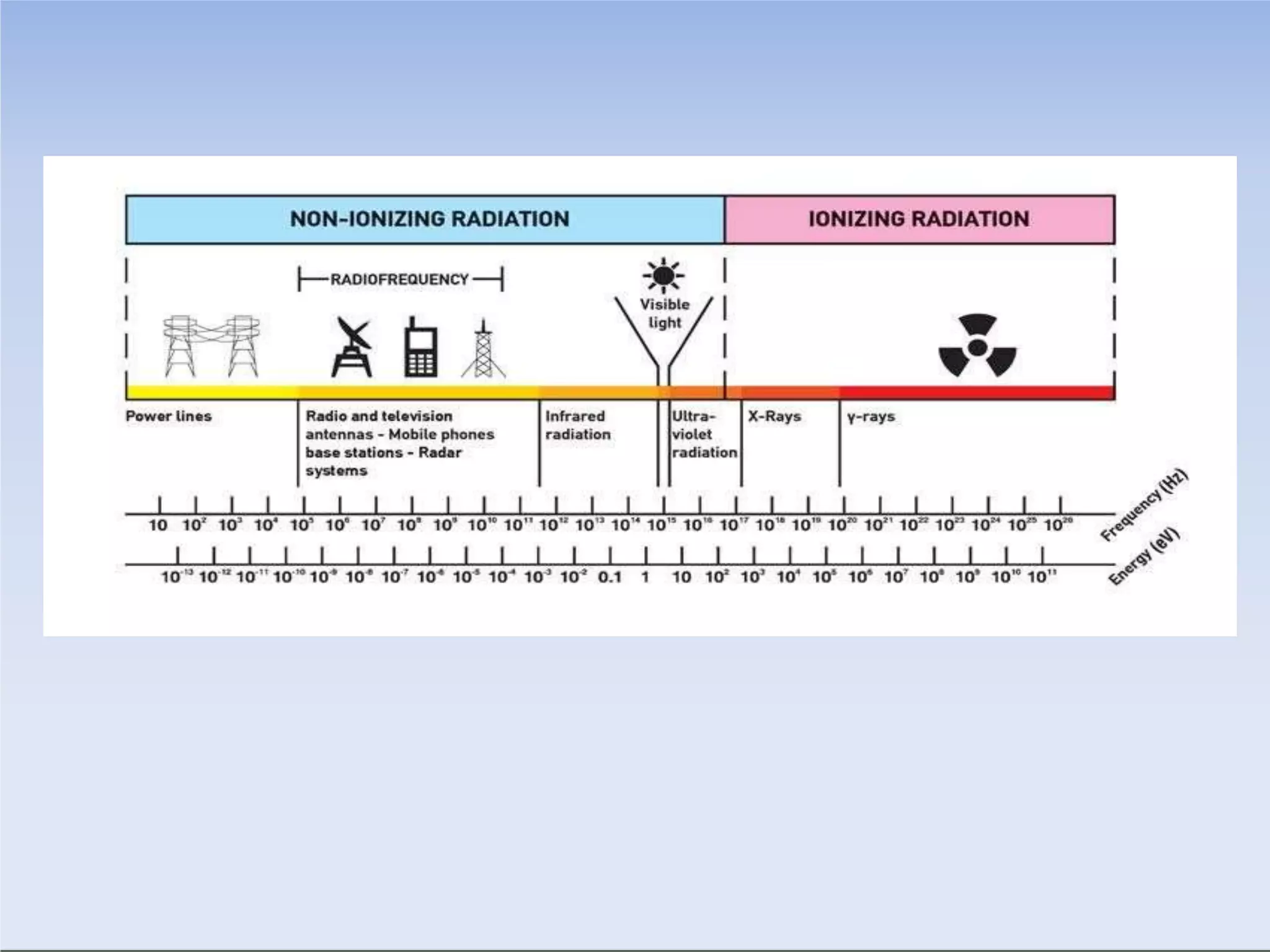
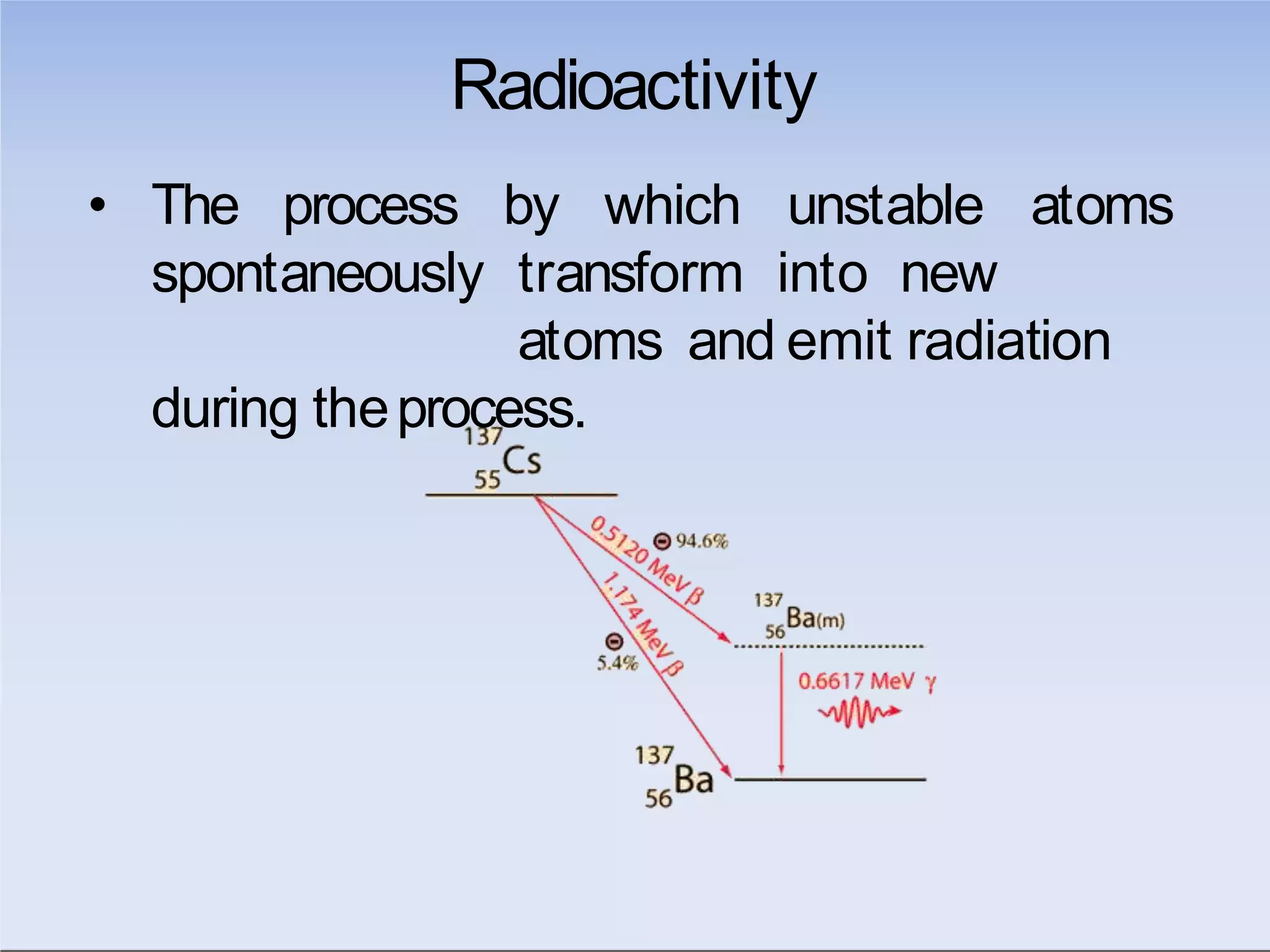
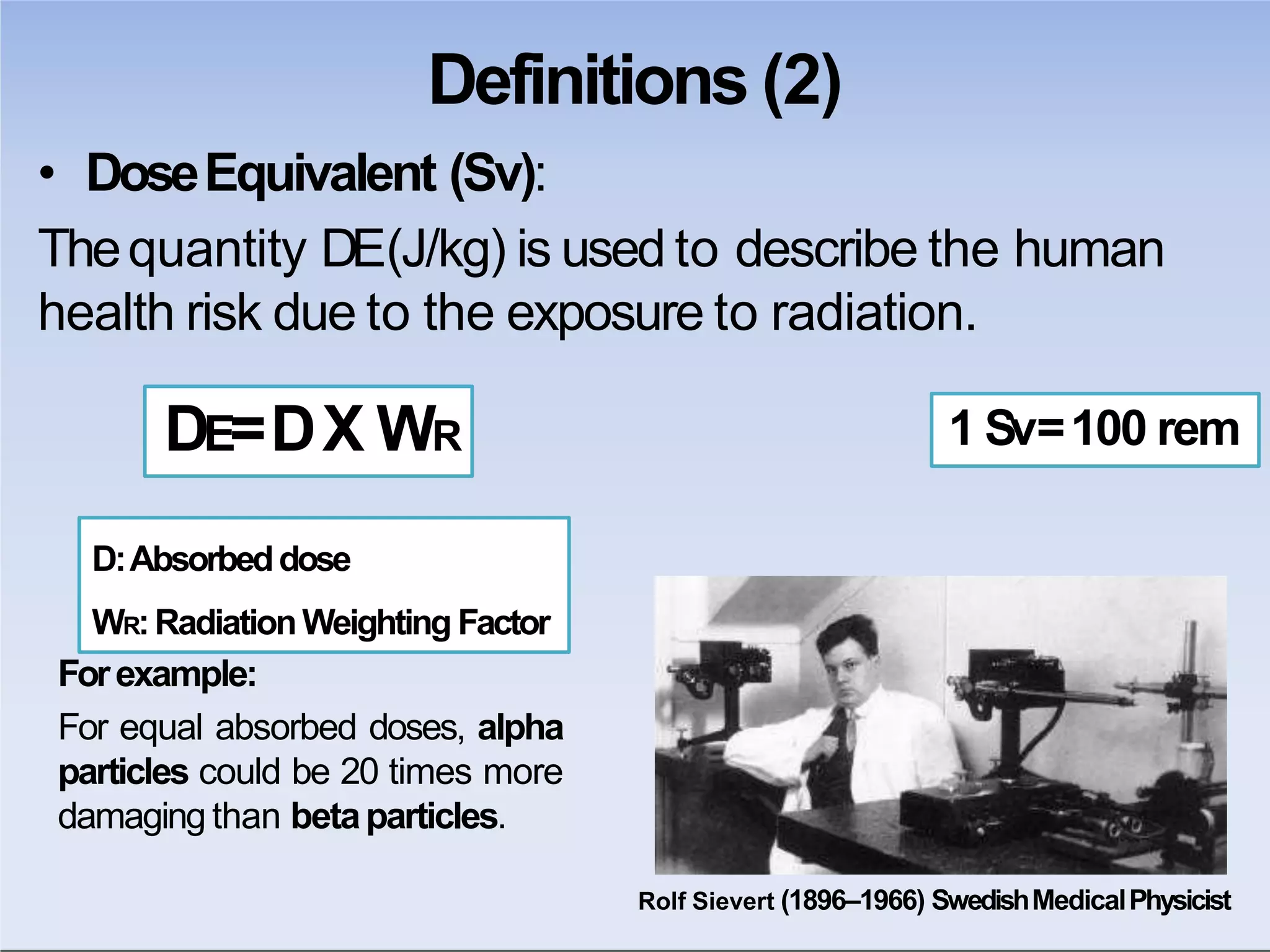
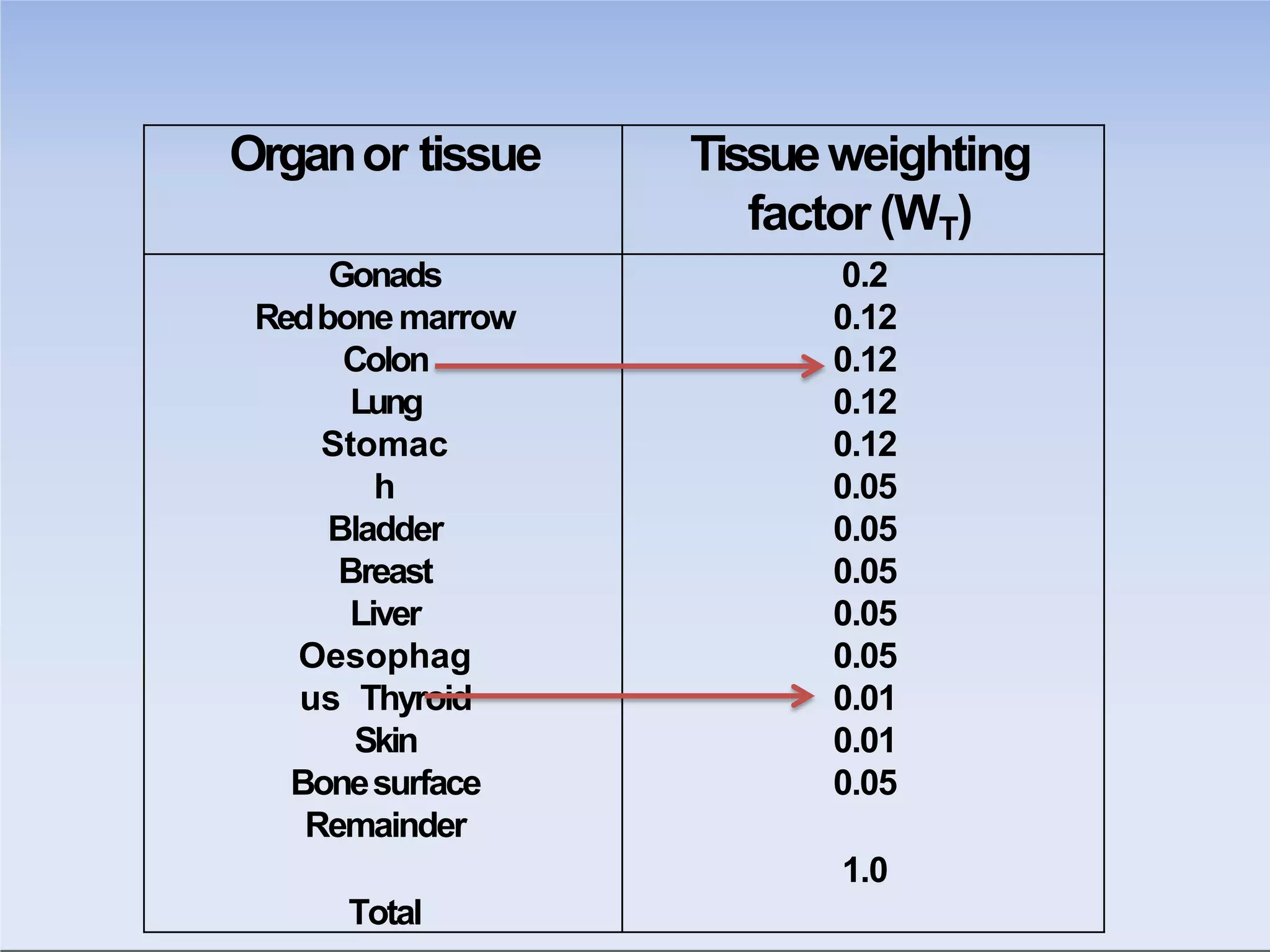
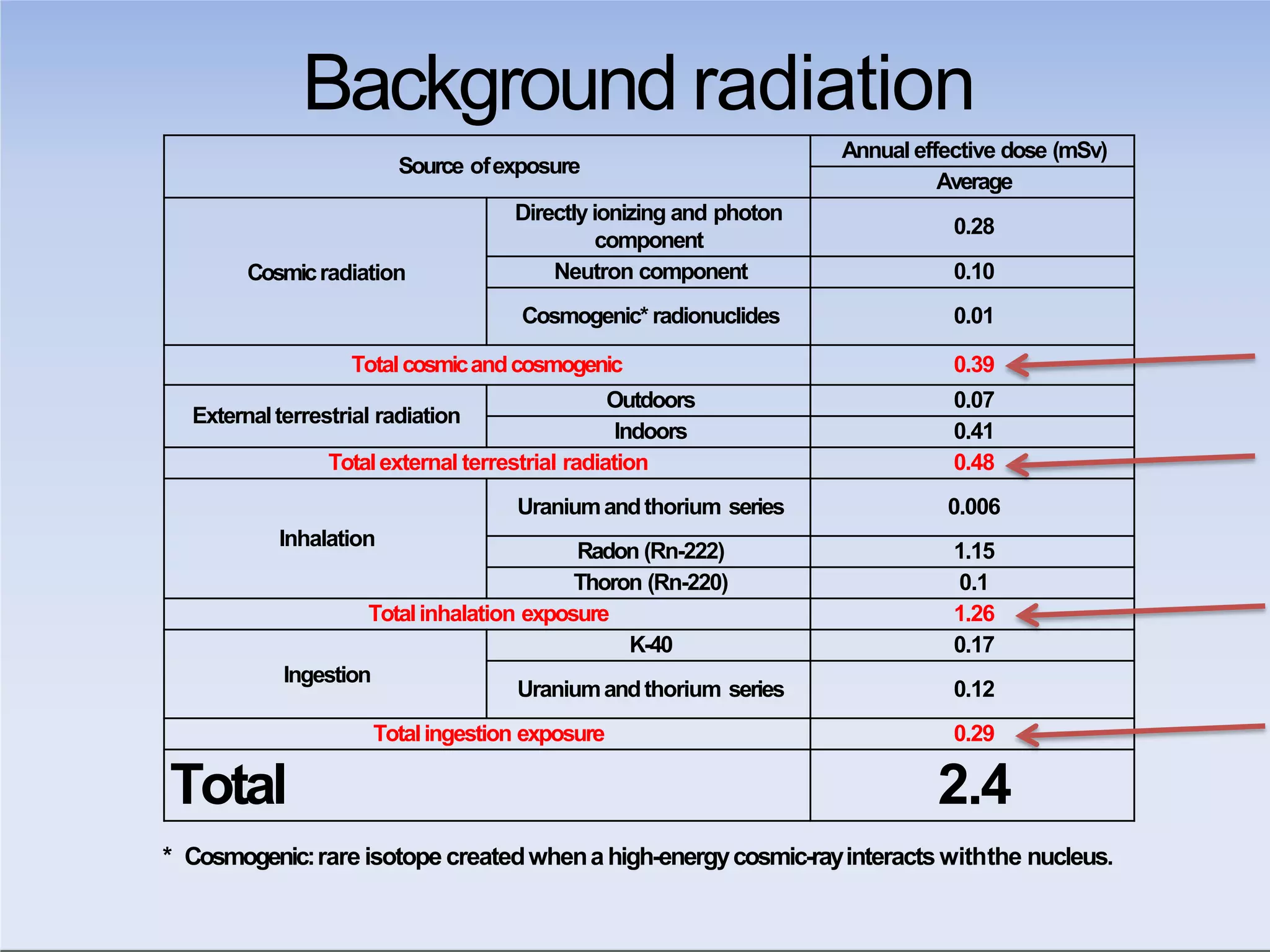
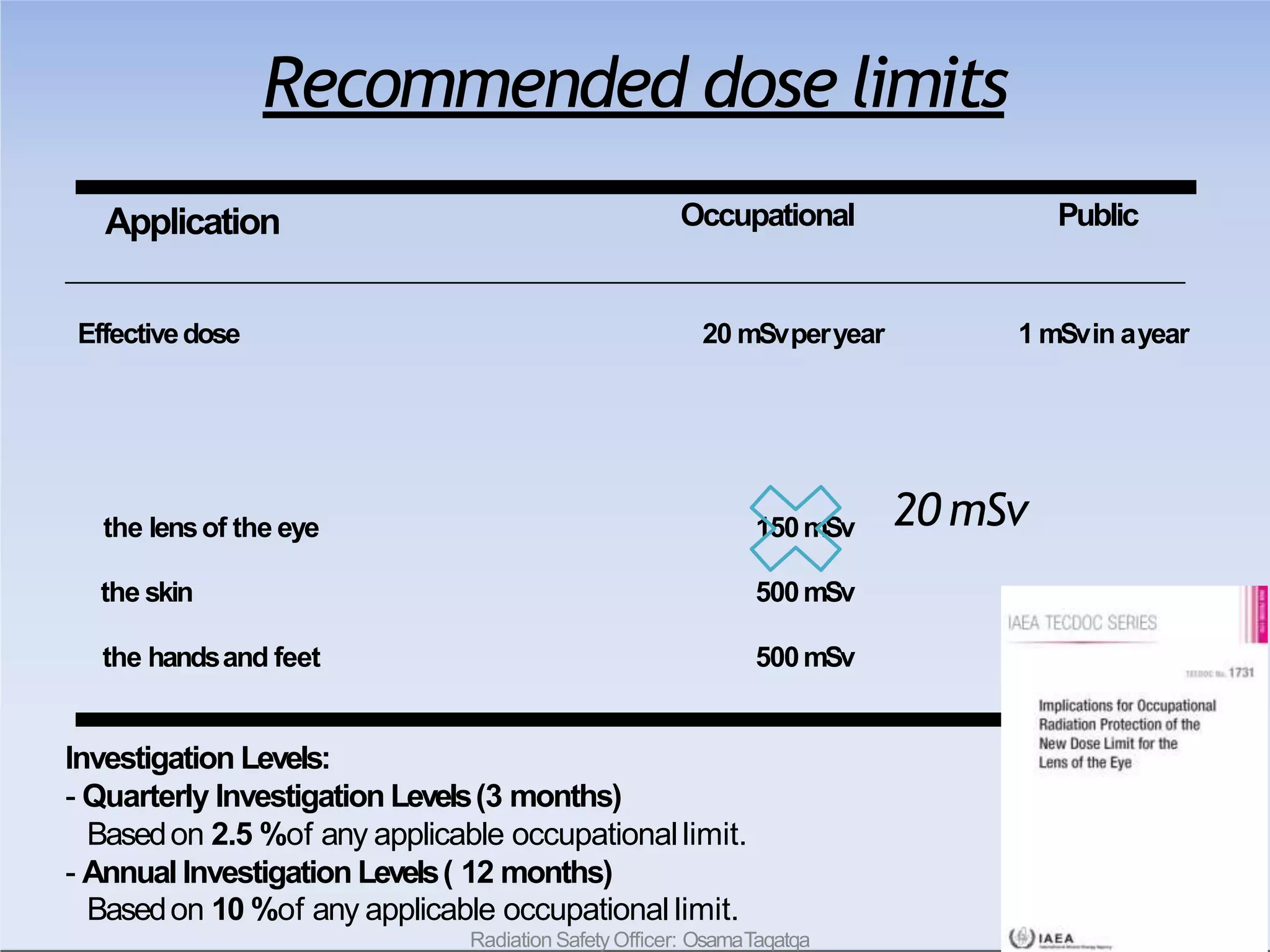
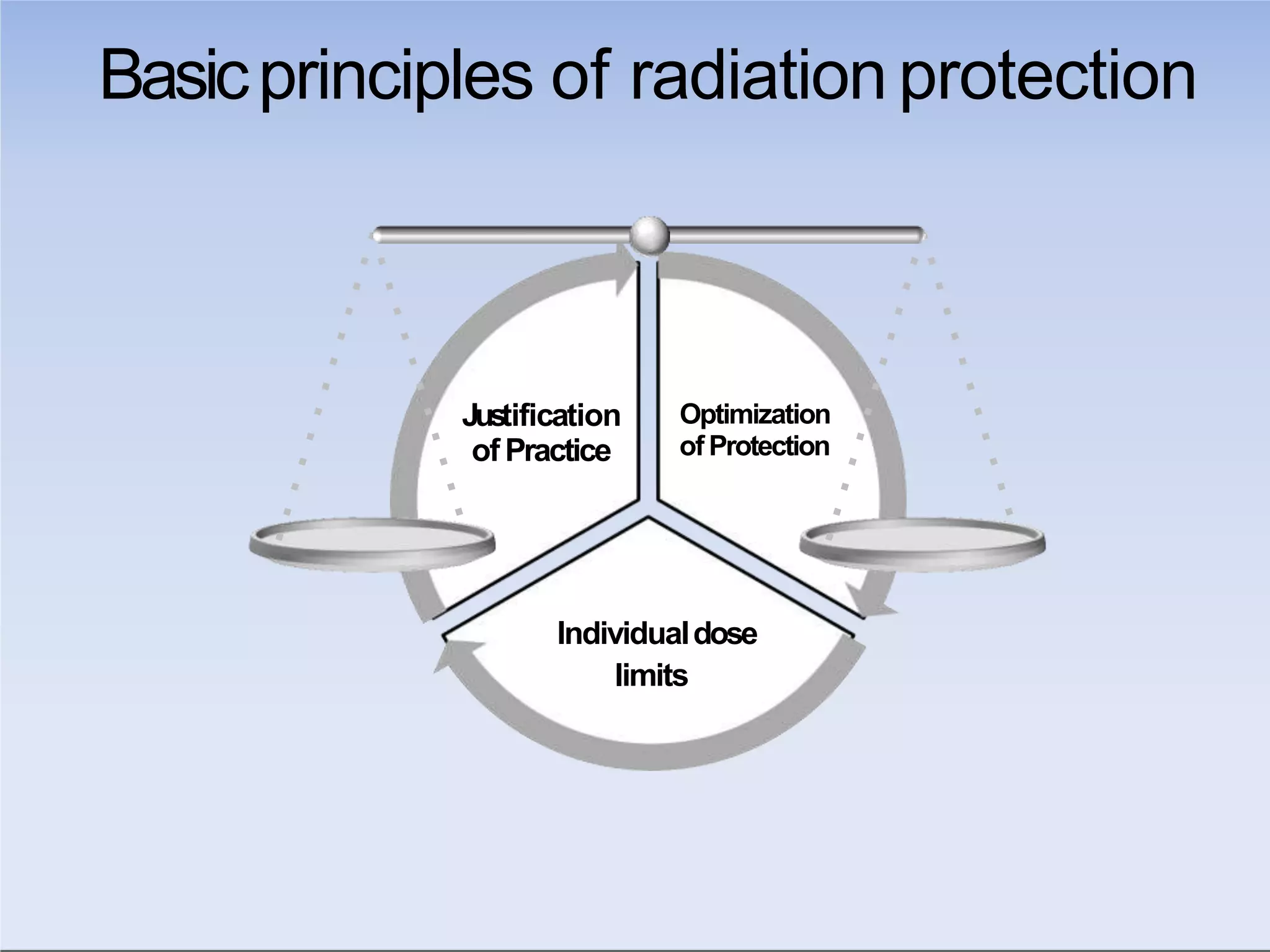
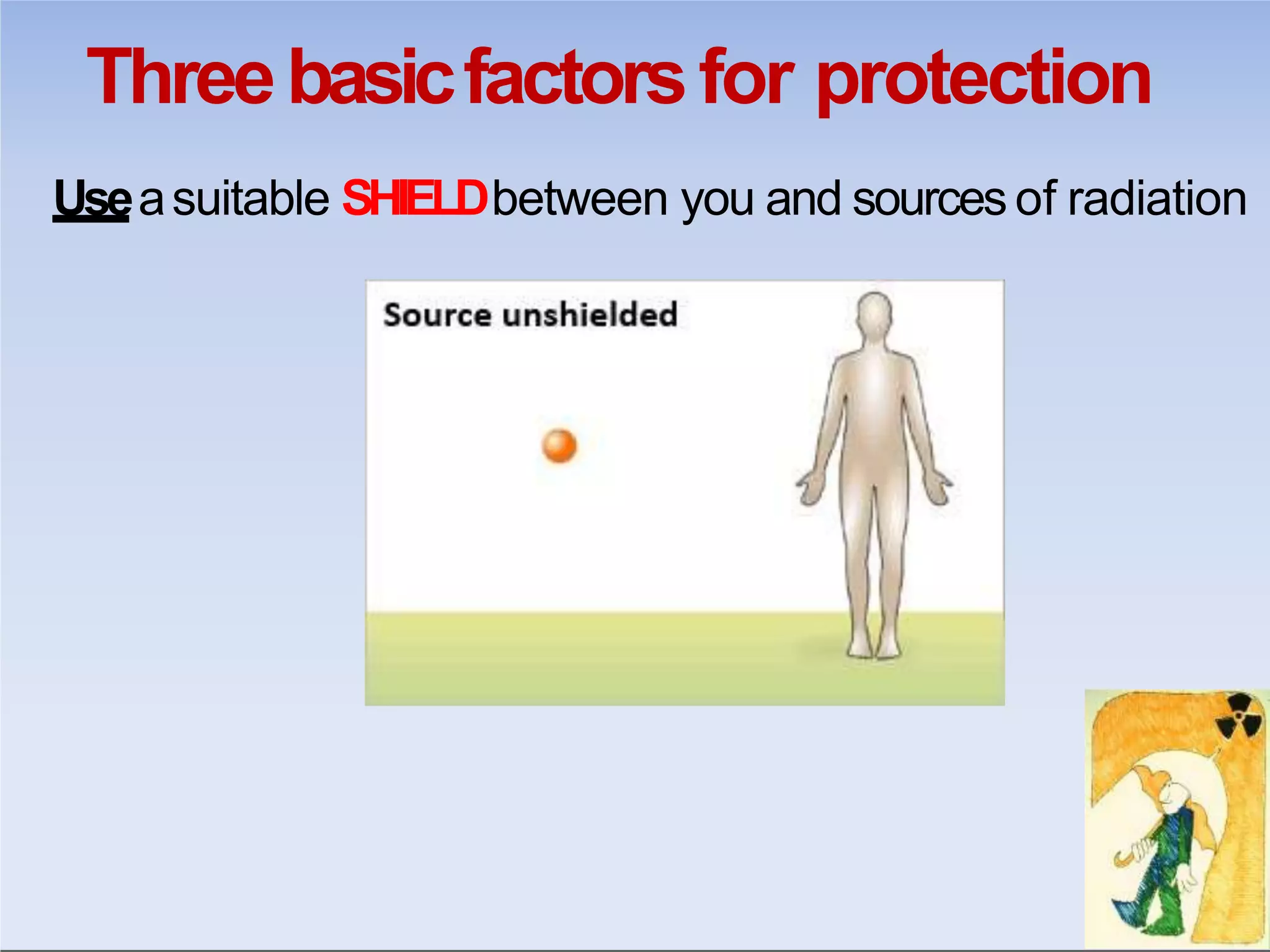
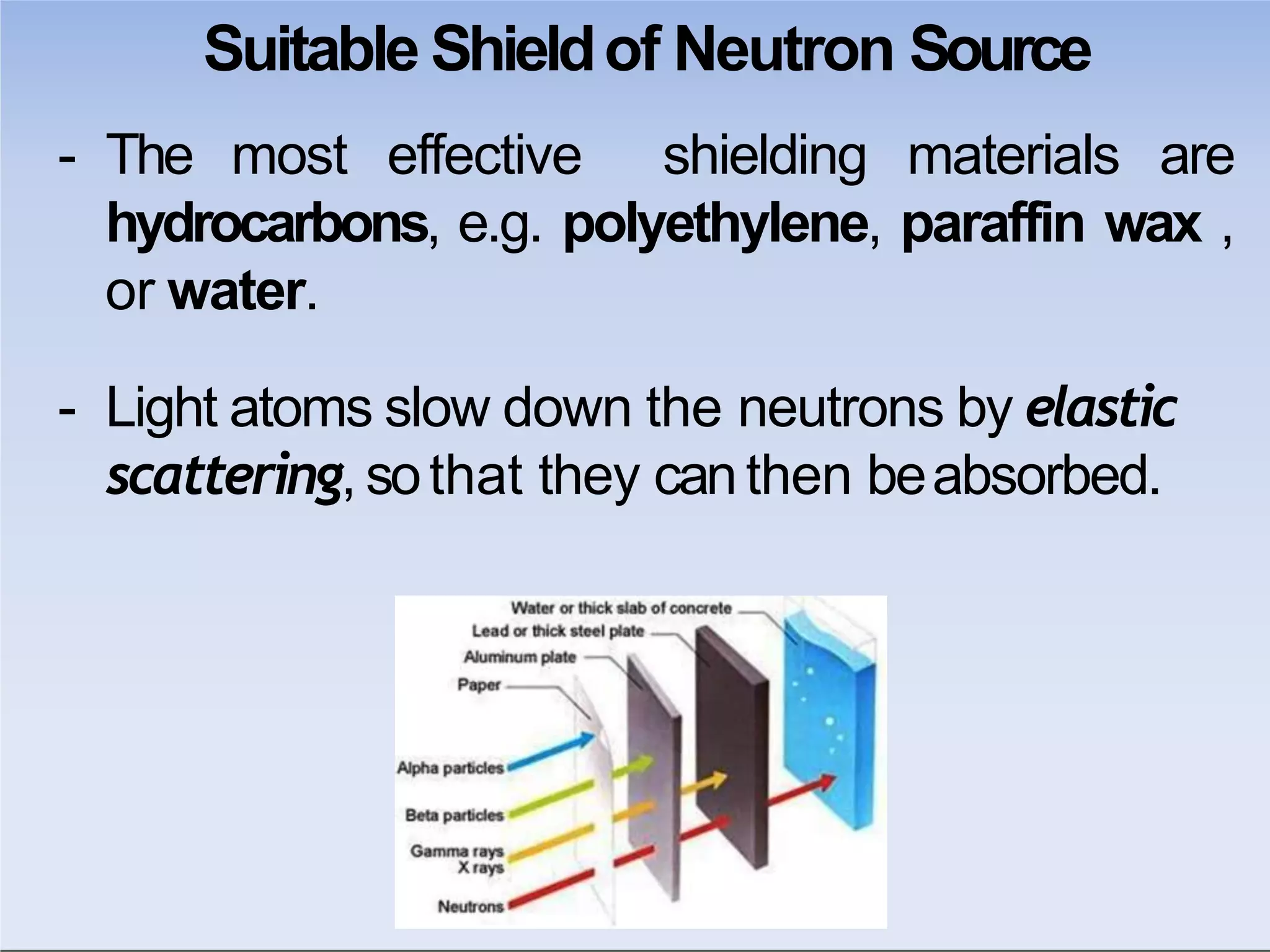
This document outlines key principles of radiation safety, including definitions of common terms like exposure, absorbed dose, and dose equivalent. It describes different types of ionizing radiation like alpha, beta, and gamma rays and their properties. Background radiation sources are identified. Recommended dose limits for occupational and public exposures are provided. The ALARA principle of maintaining radiation exposures as low as reasonably achievable is introduced. Common radiation safety equipment and signage are depicted.