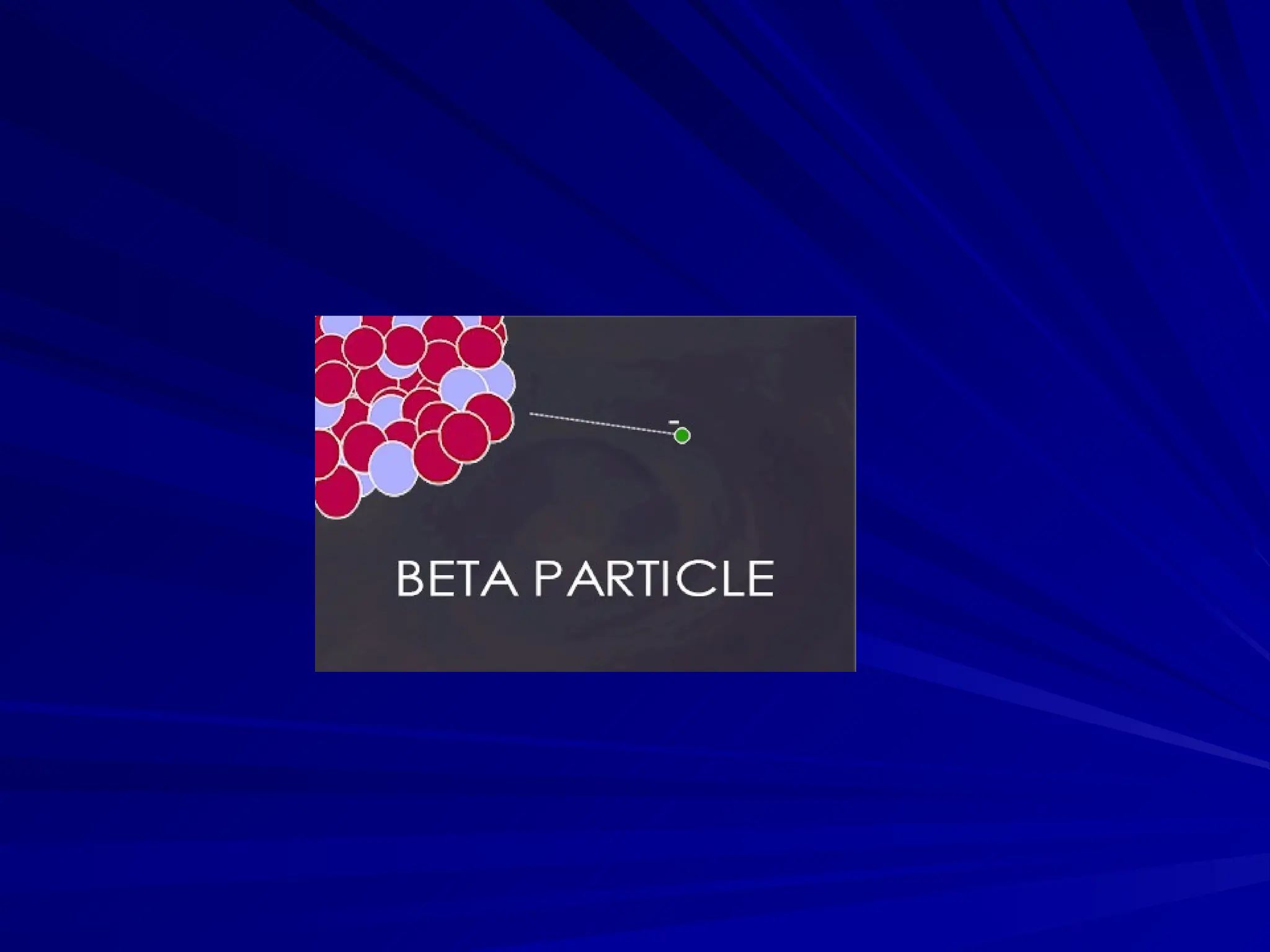
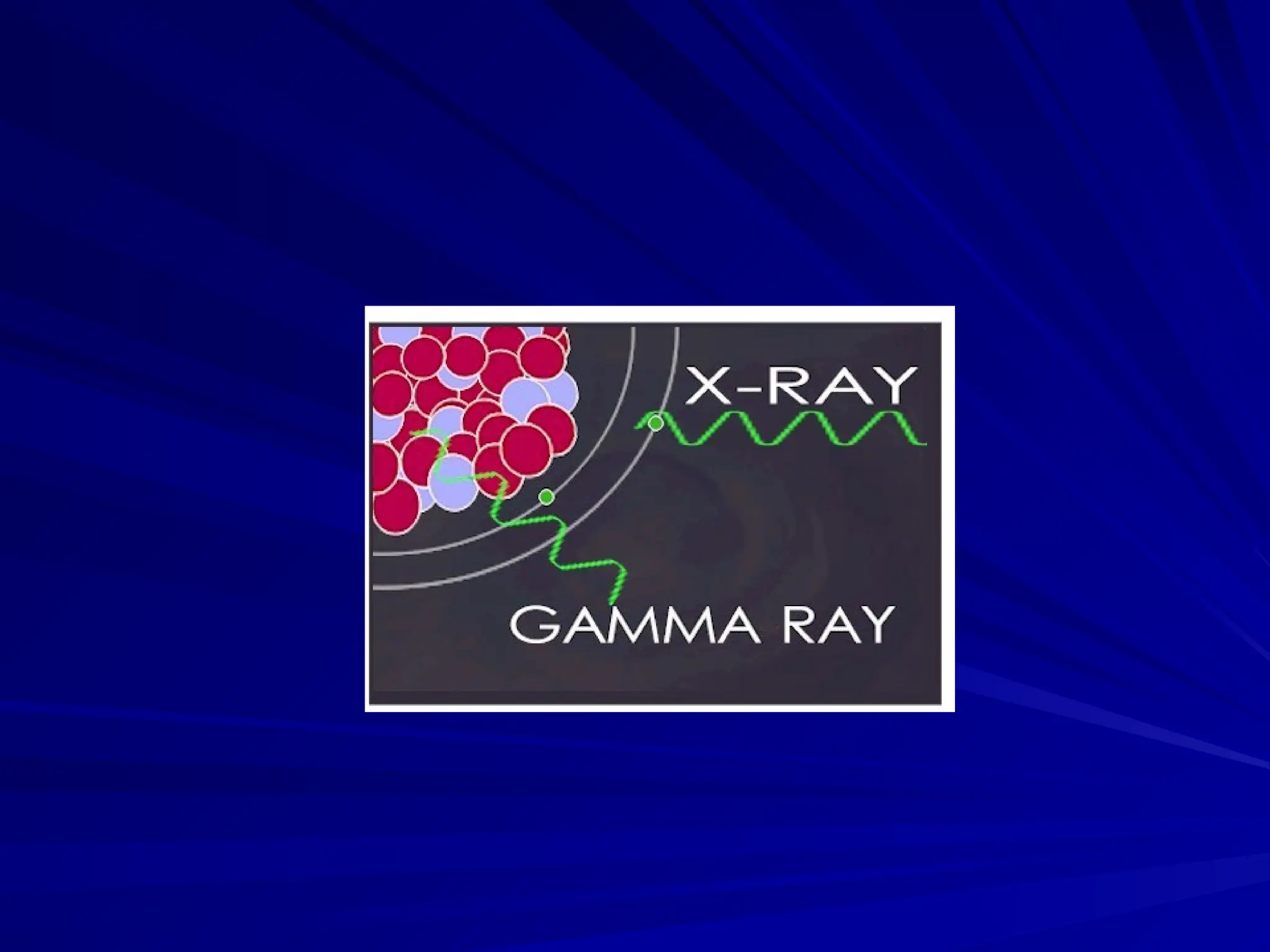
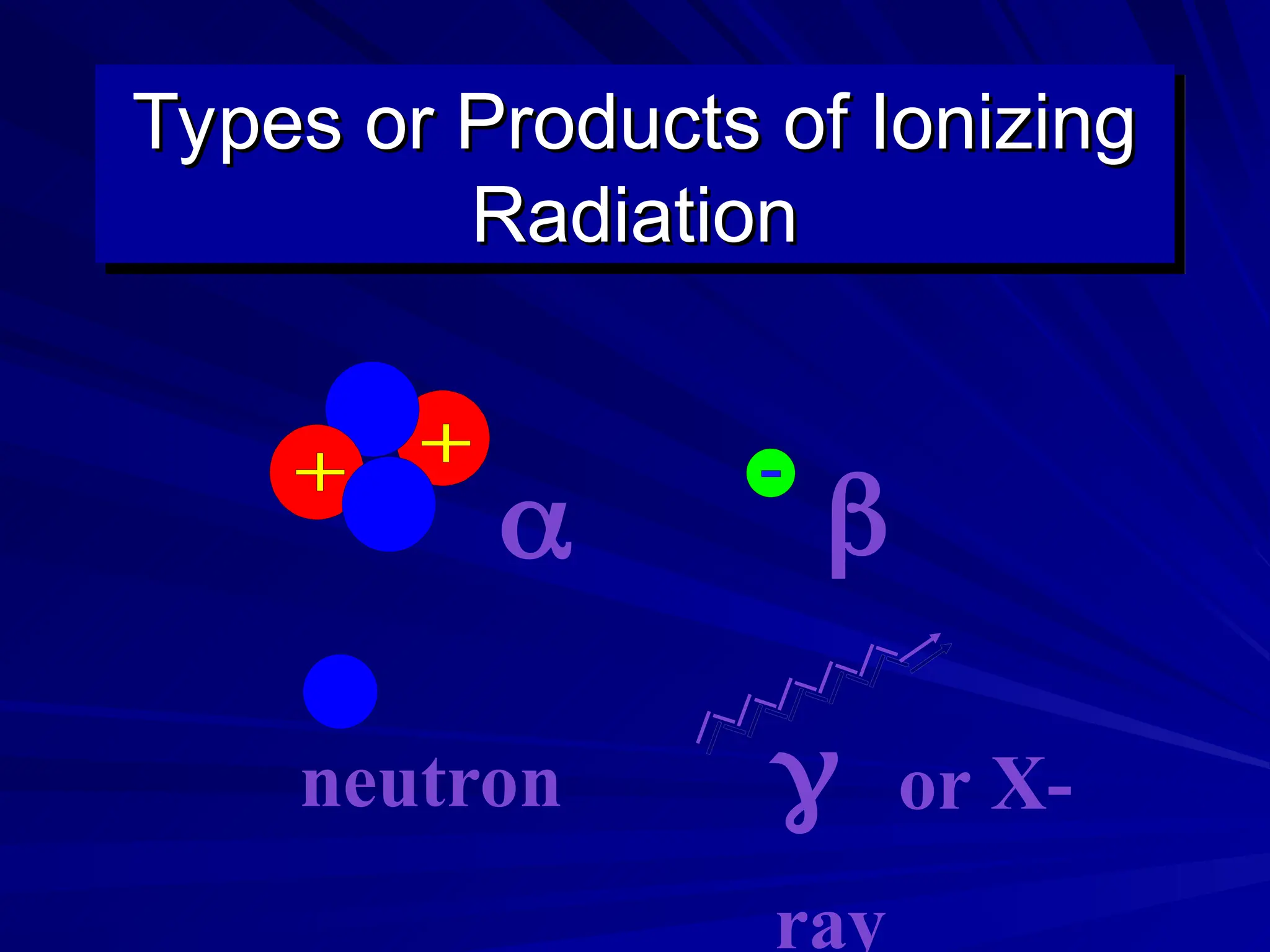
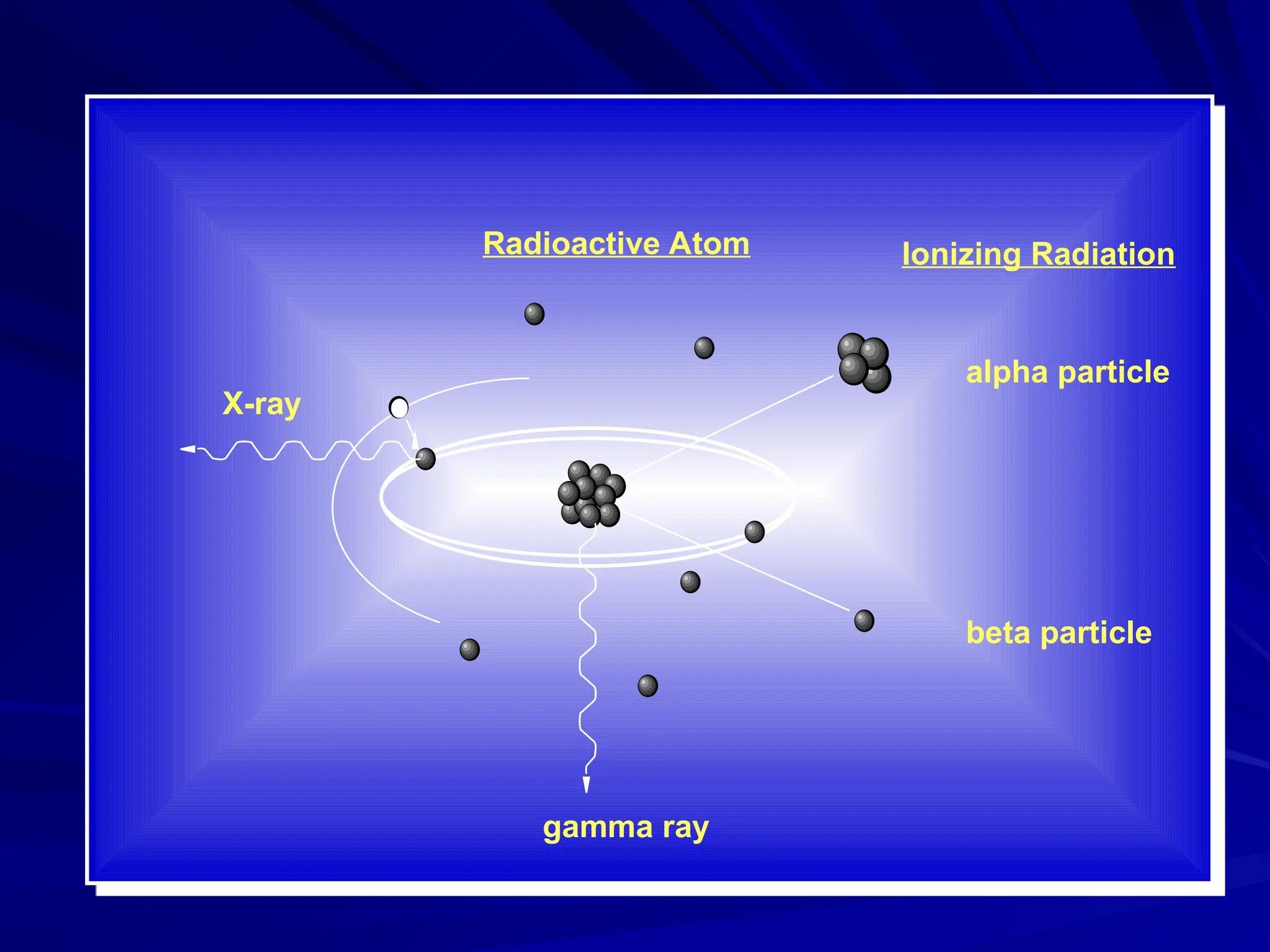
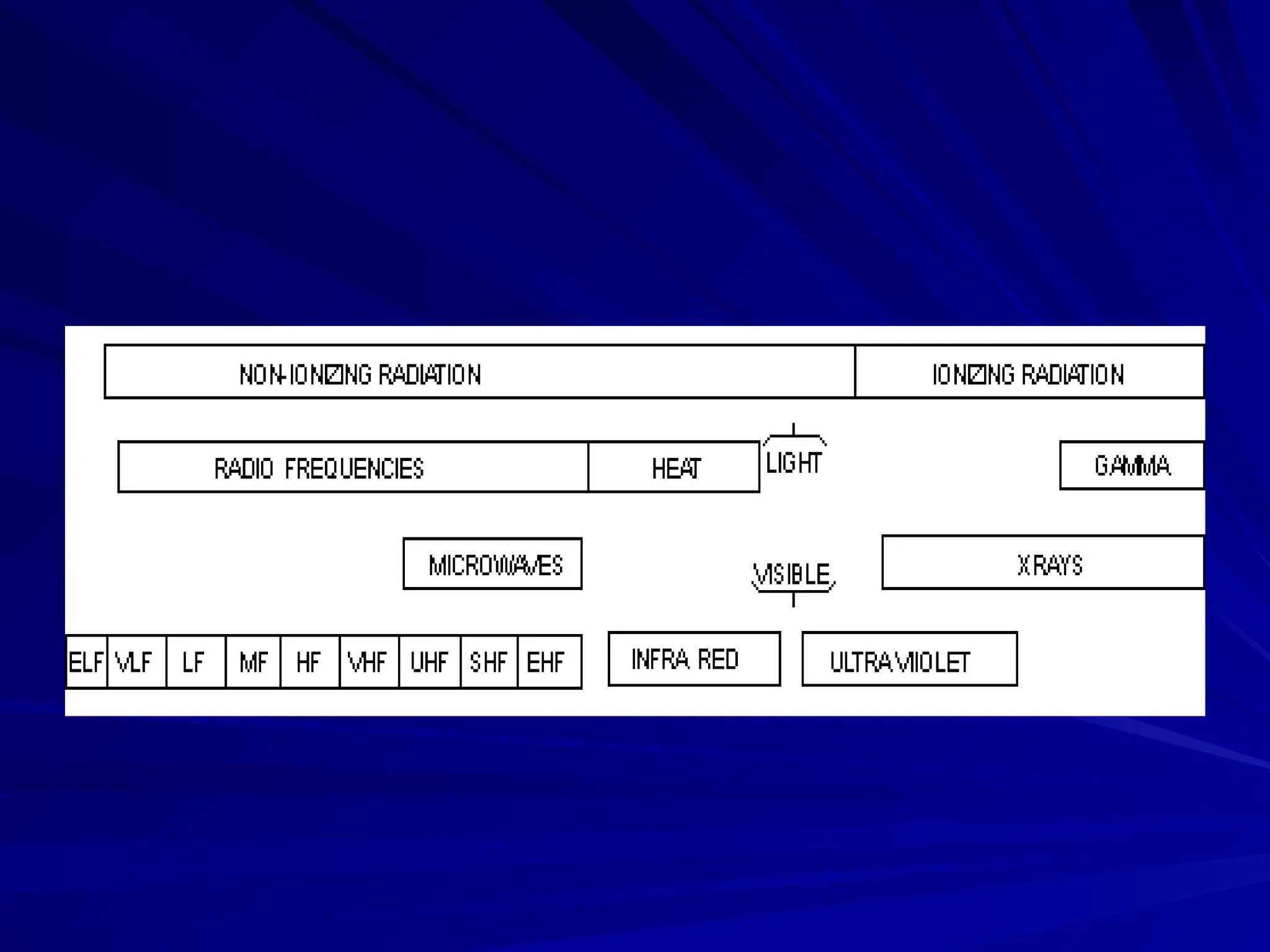
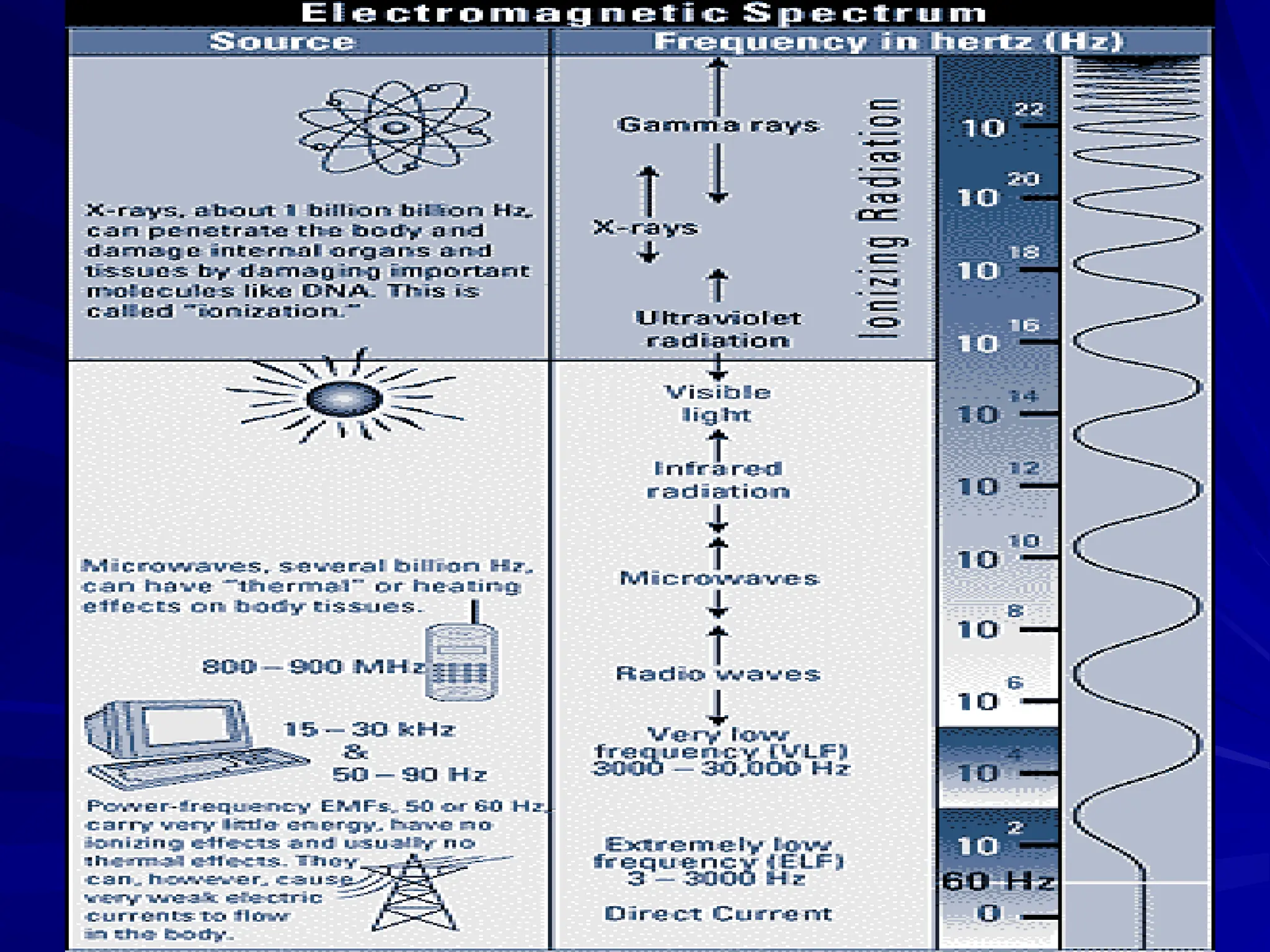
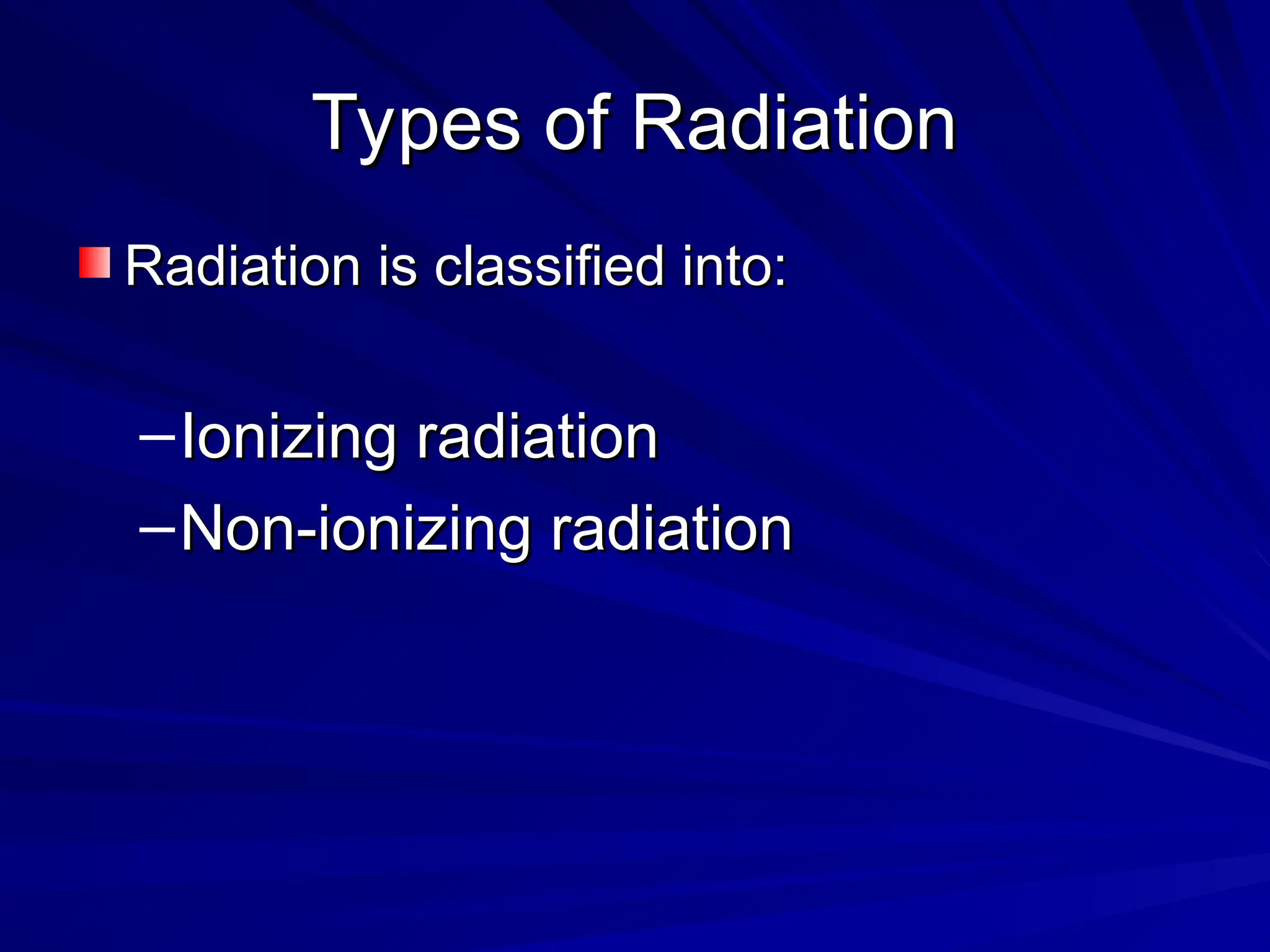
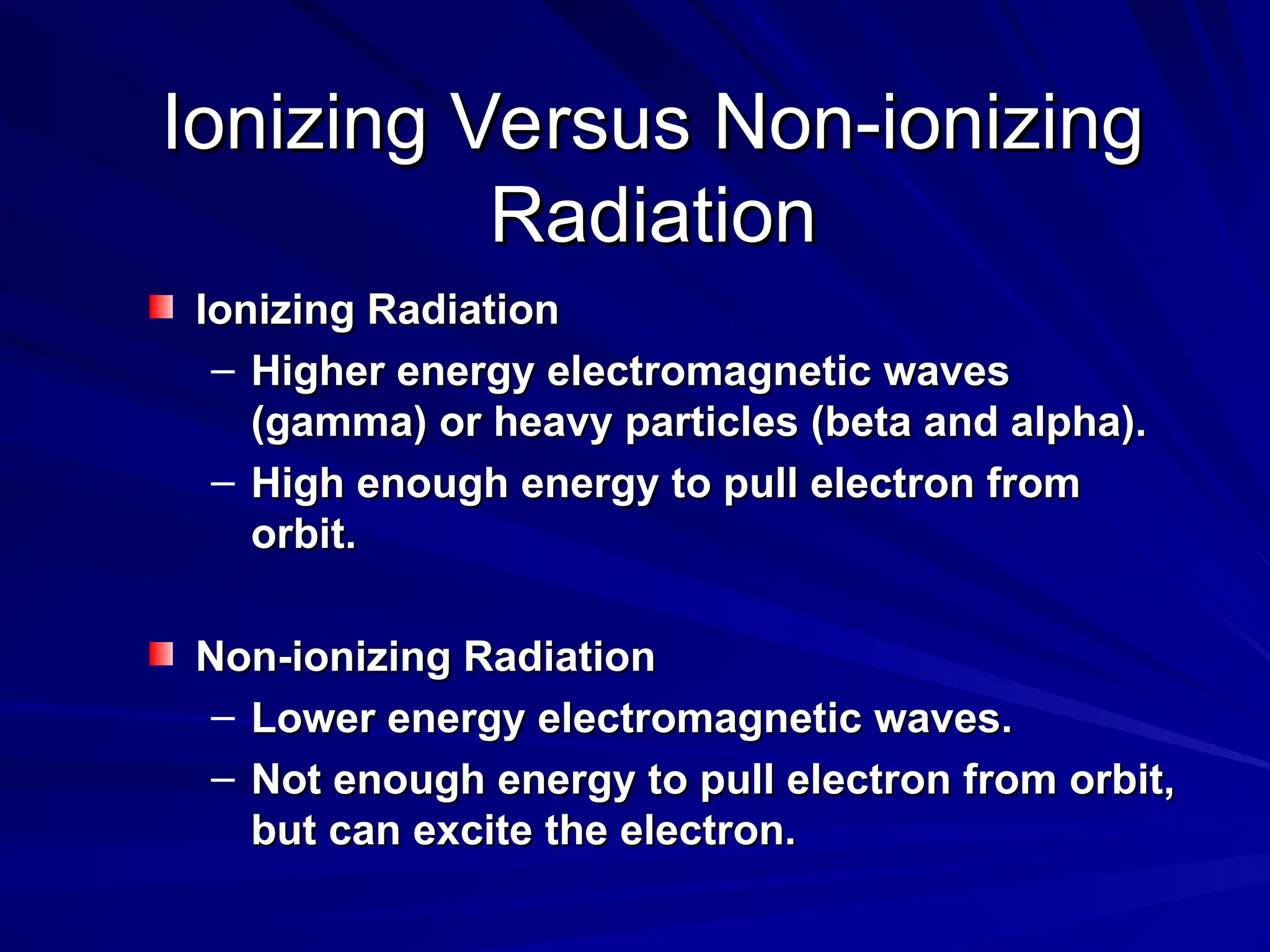
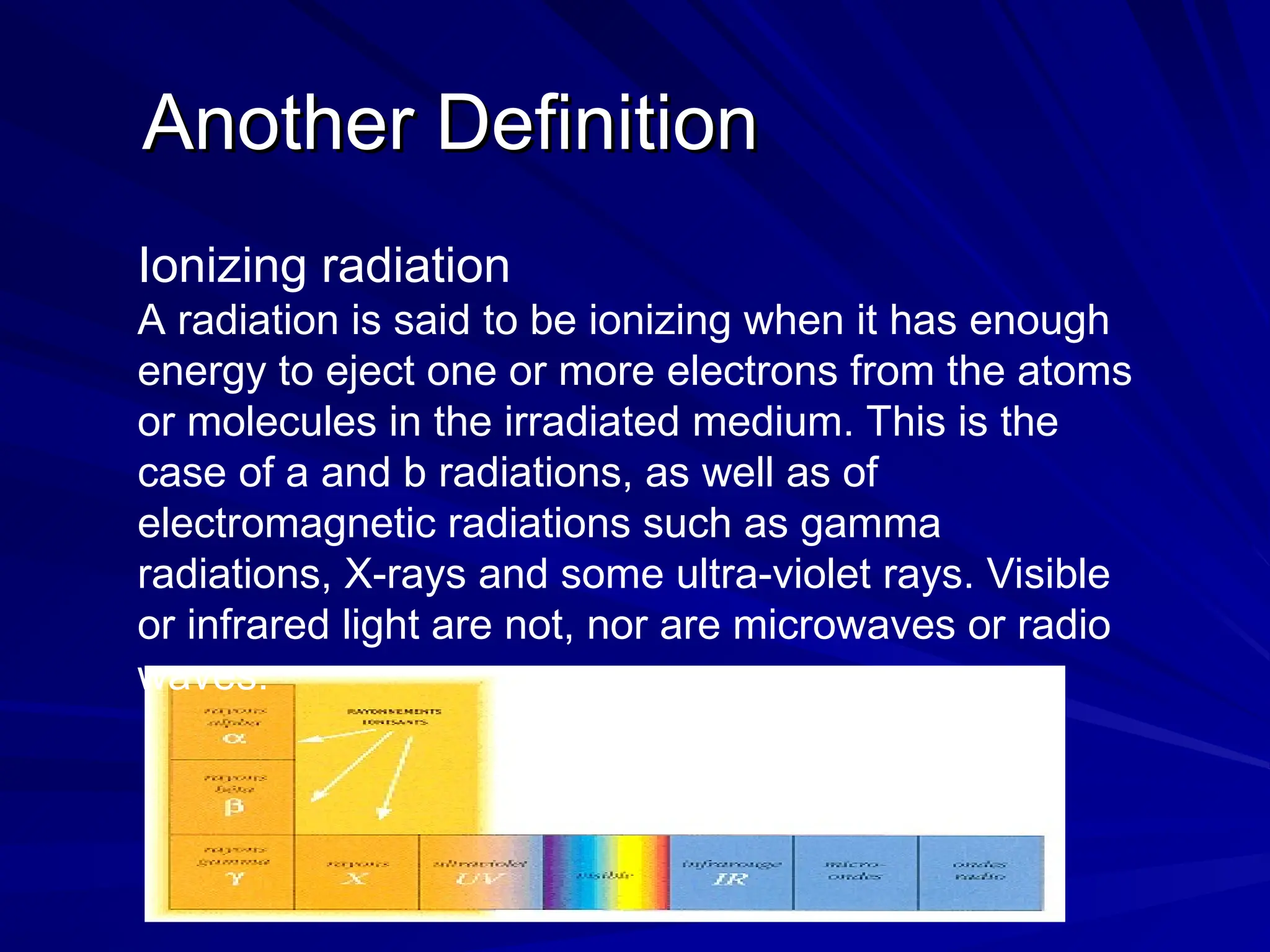
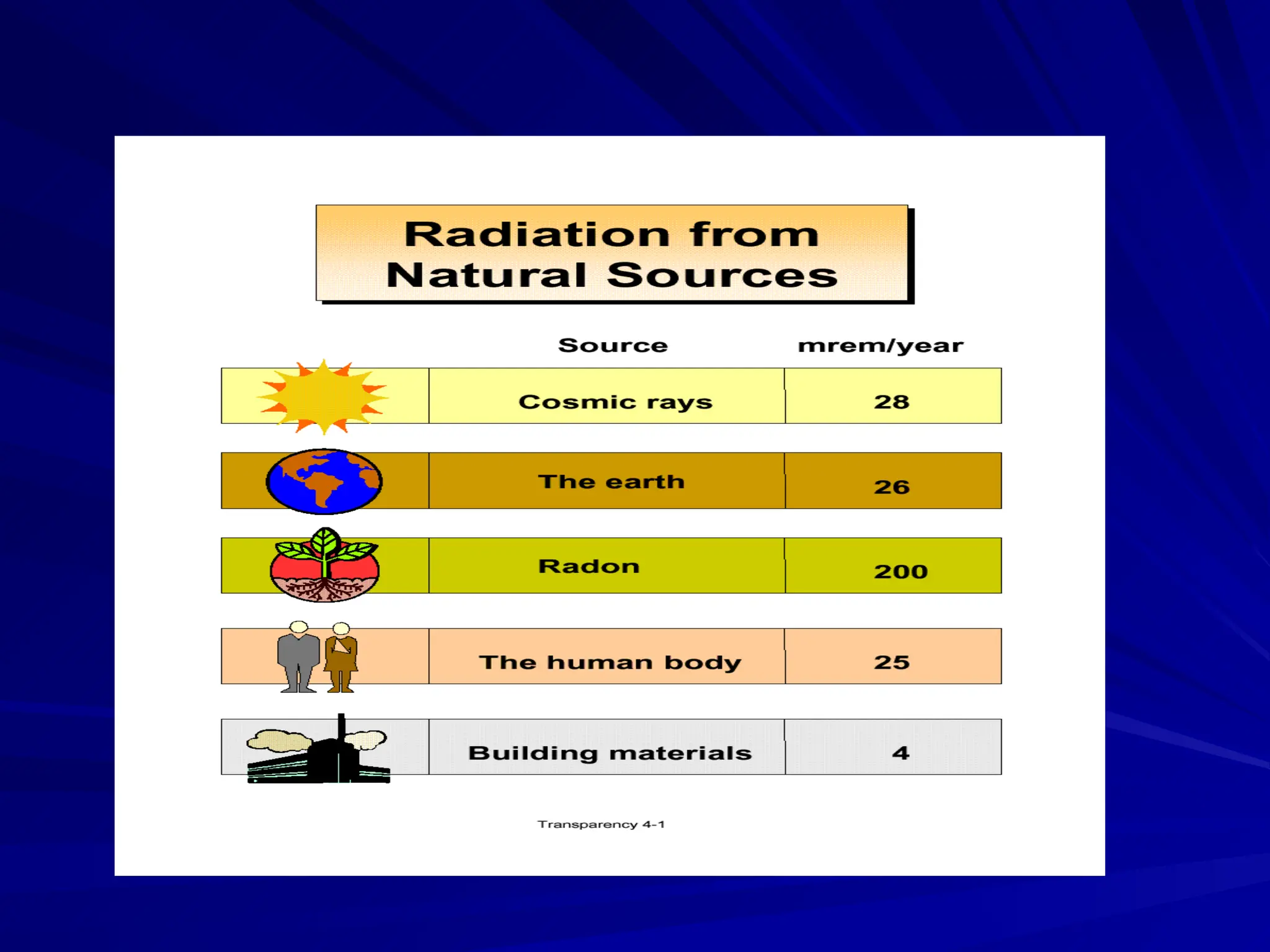
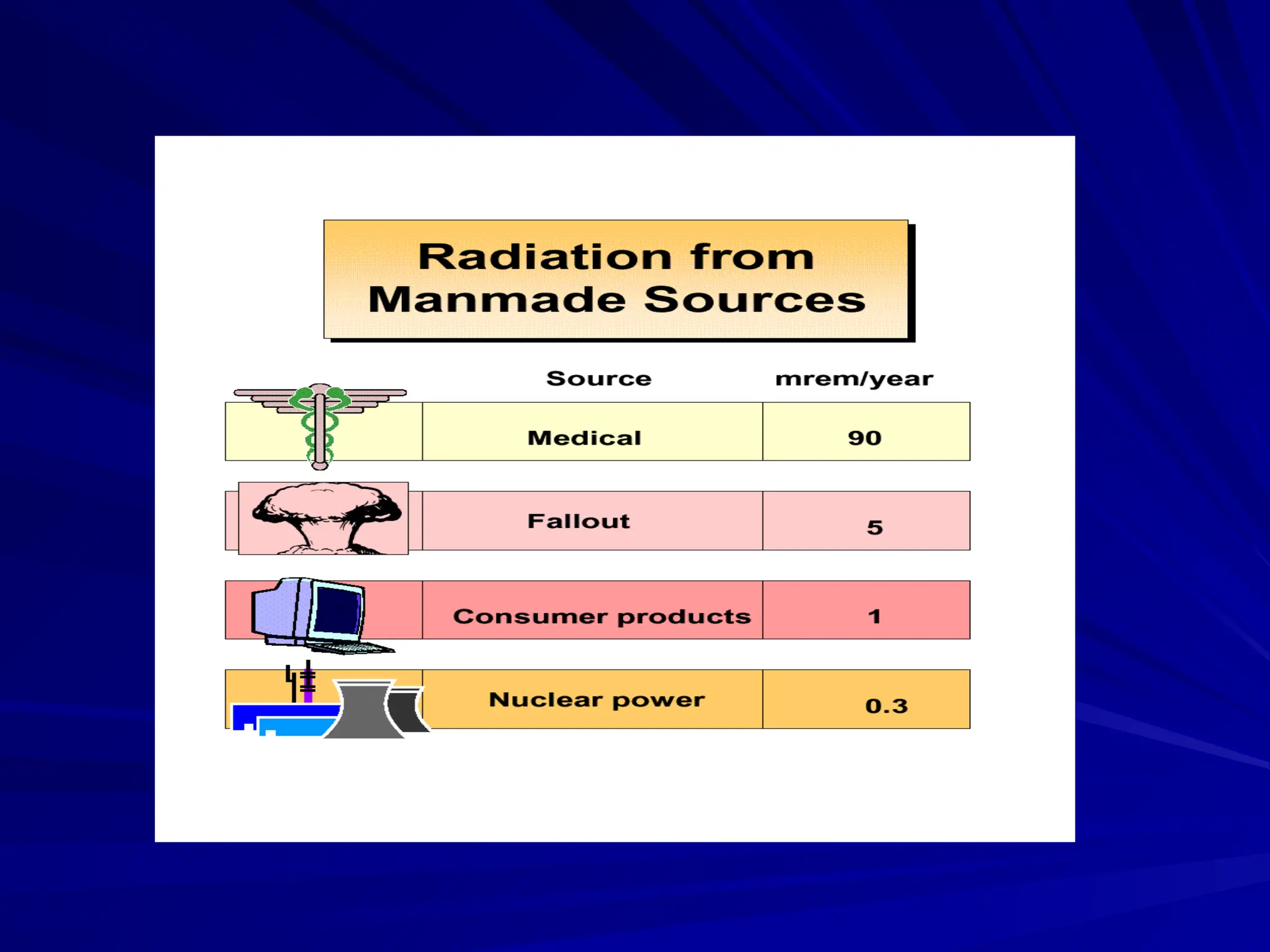
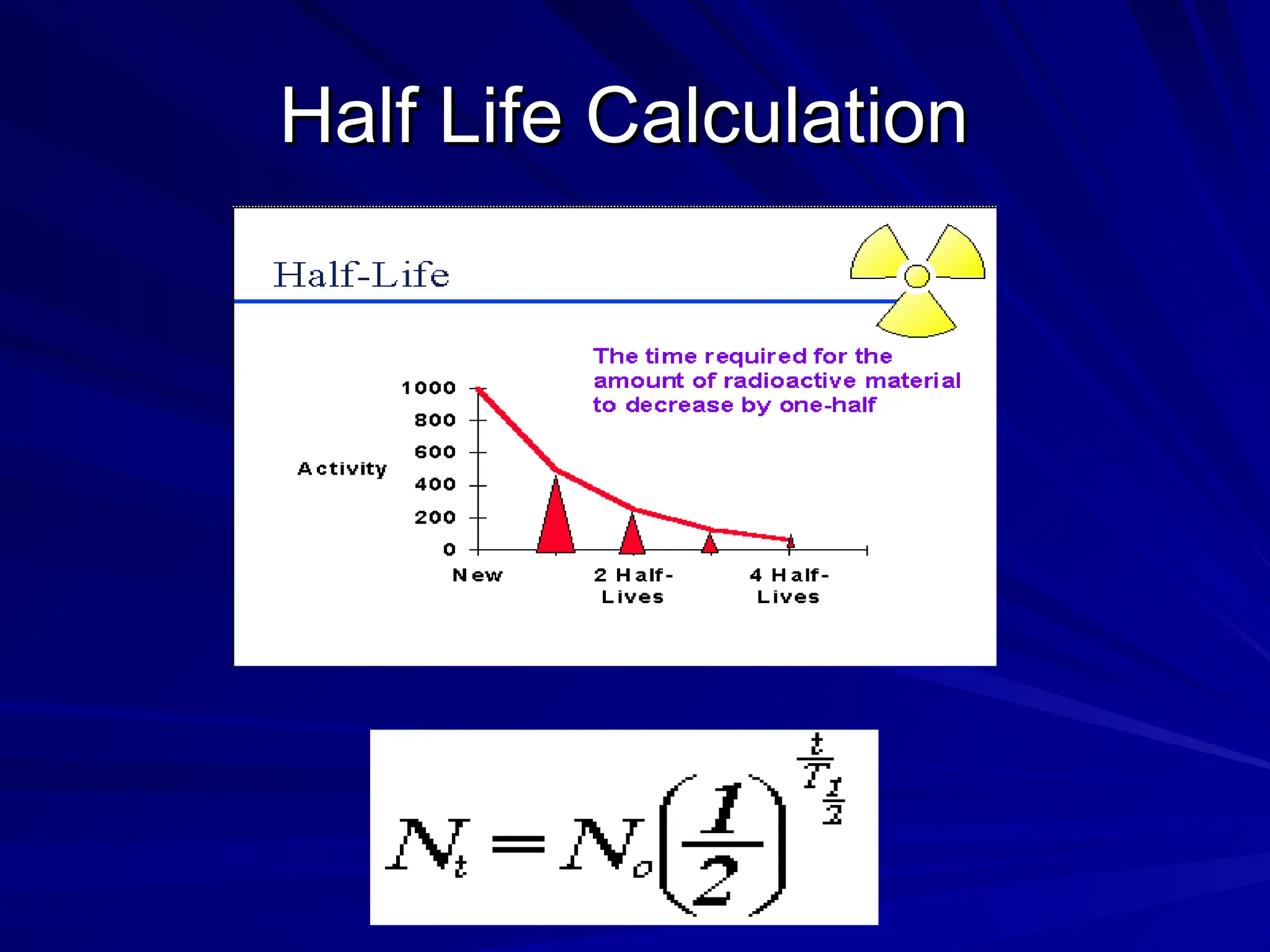
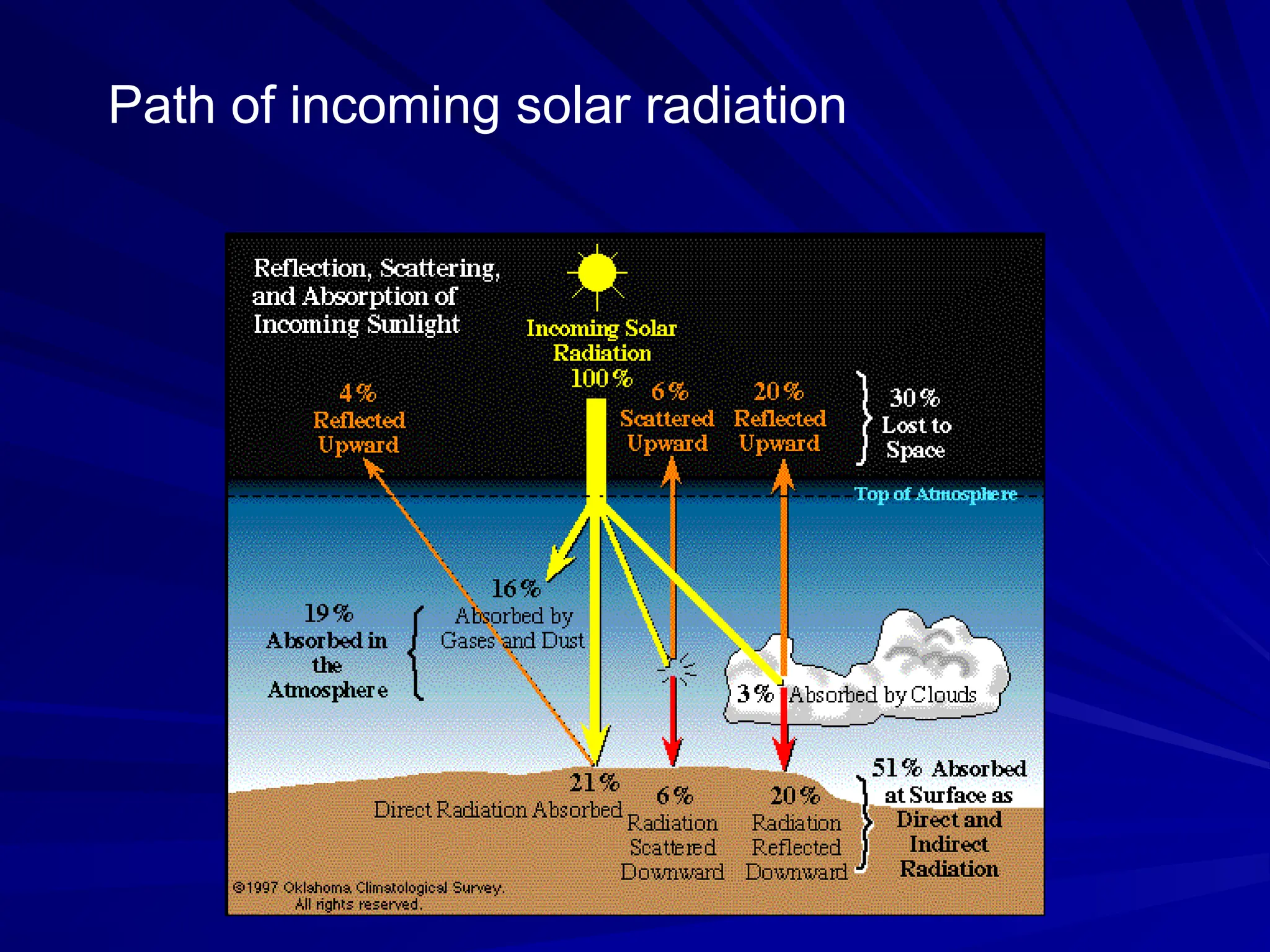
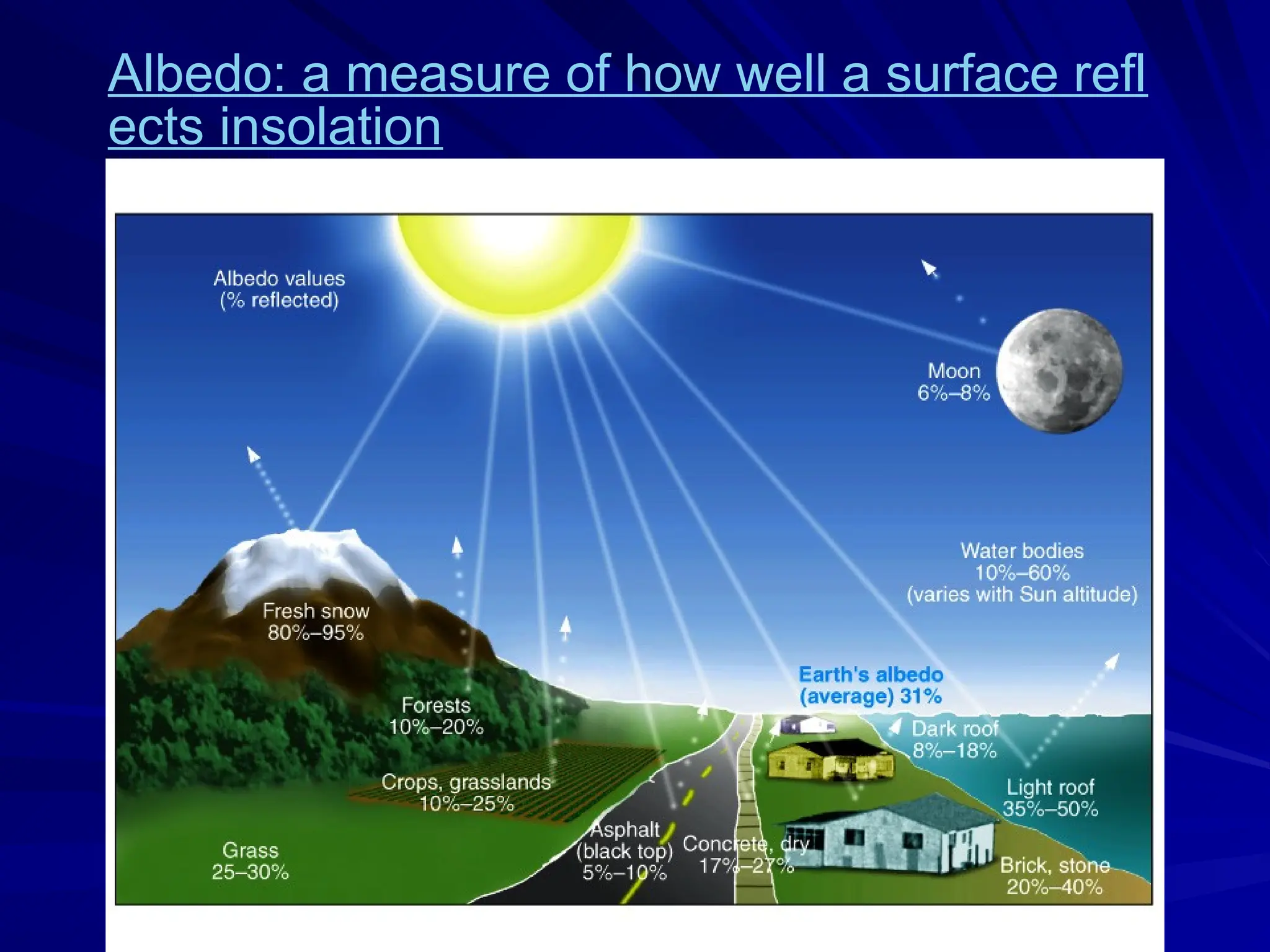
The document provides an extensive overview of radiation, detailing its definitions, types, and interactions, particularly focusing on ionizing and non-ionizing radiation. It explains the characteristics and biological effects of various types of radiation, including alpha particles, beta particles, gamma rays, and x-rays, while also addressing exposure limits and health effects related to radiation. Additionally, it outlines safety measures and the quantification of exposure and radioactive decay, emphasizing the importance of understanding radiation in terms of both everyday health and nuclear physics.