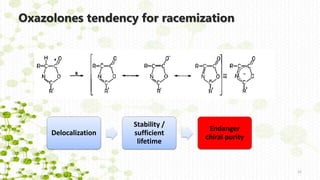
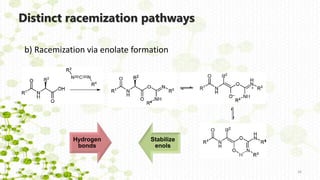
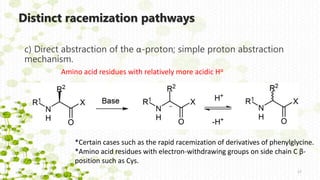
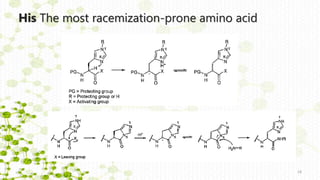
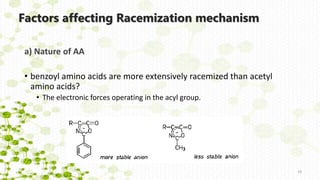
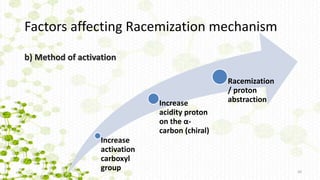
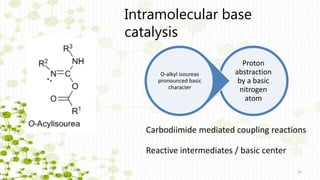
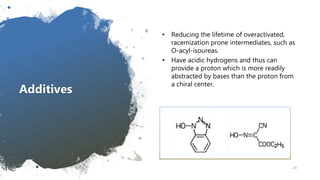
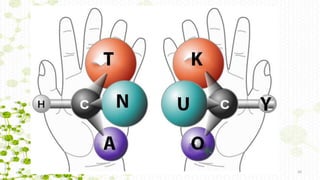
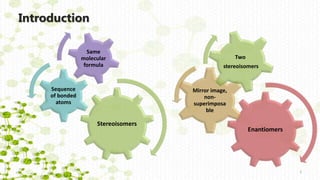
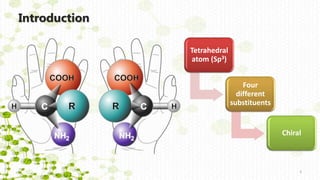
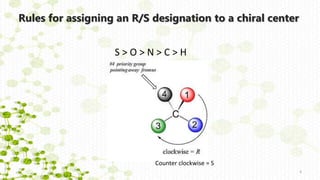
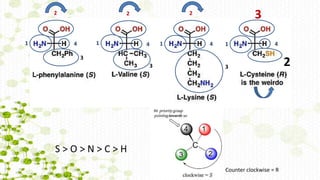
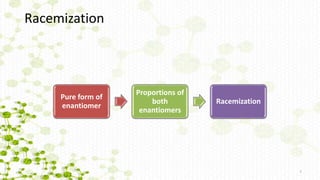
This document discusses racemization in peptide synthesis. It introduces the concepts of stereoisomers, enantiomers, and chiral centers. It then discusses factors that can lead to racemization, such as the amino acid nature, activation method, solvent used, amount and character of the base, and presence of additives. Specific amino acids like histidine are highlighted as being more prone to racemization. Different racemization pathways are proposed, including those involving azlactone intermediates, enolate formation, and direct abstraction of the alpha proton. Methods to reduce racemization, such as using non-polar solvents or bulkier bases, are also covered.





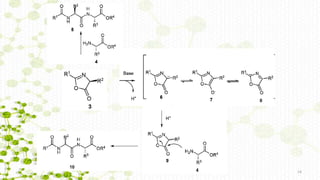
![a) Racemization through azlactones [5(4 H)-oxazolones].
Distinct racemization pathways
13
Nα group of the activated amino acid is acylated](https://image.slidesharecdn.com/racemizationothman-181015184457/85/Racemization-in-peptide-synthesis-13-320.jpg)