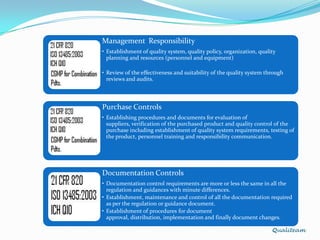
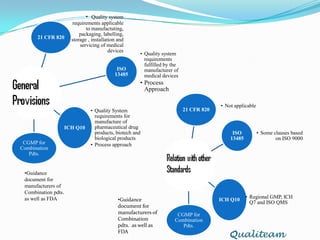
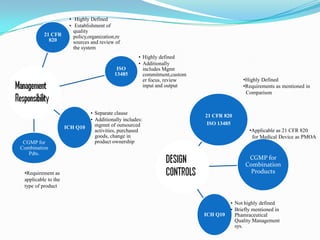
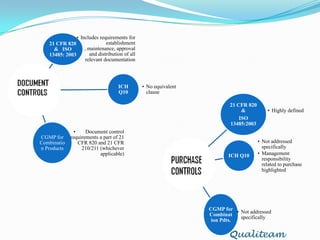
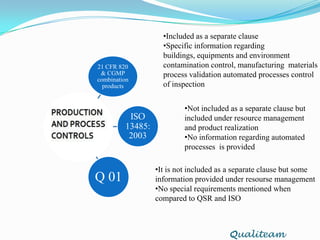
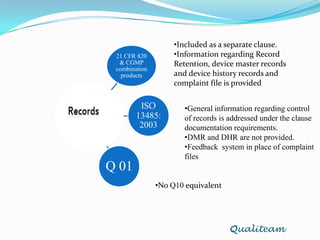
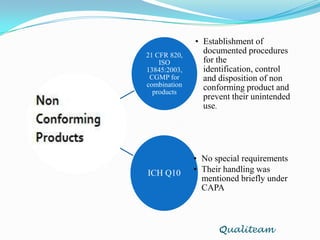
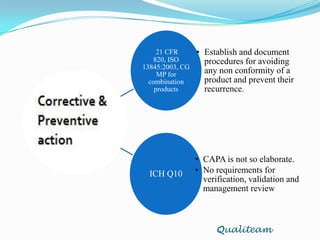
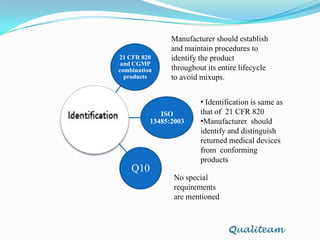
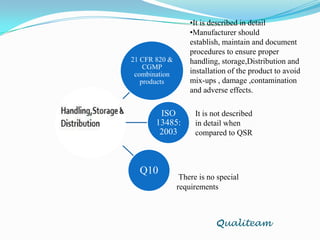
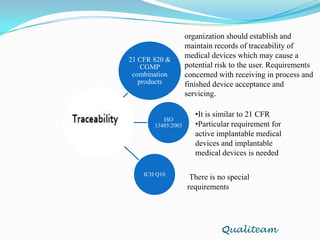
The document compares and contrasts quality systems requirements from 21 CFR 820, ISO 13845:2003, ICH Q10, and CGMP for combination products. It finds that 21 CFR 820, ISO 13845:2003, and CGMP show similarities in documentation control, design control, management responsibilities, corrective and preventive actions, and handling of nonconforming products. However, the standards also differ in areas like management responsibility requirements, additional clauses in ICH Q10, and additional drug-related requirements for combination products under CGMP.