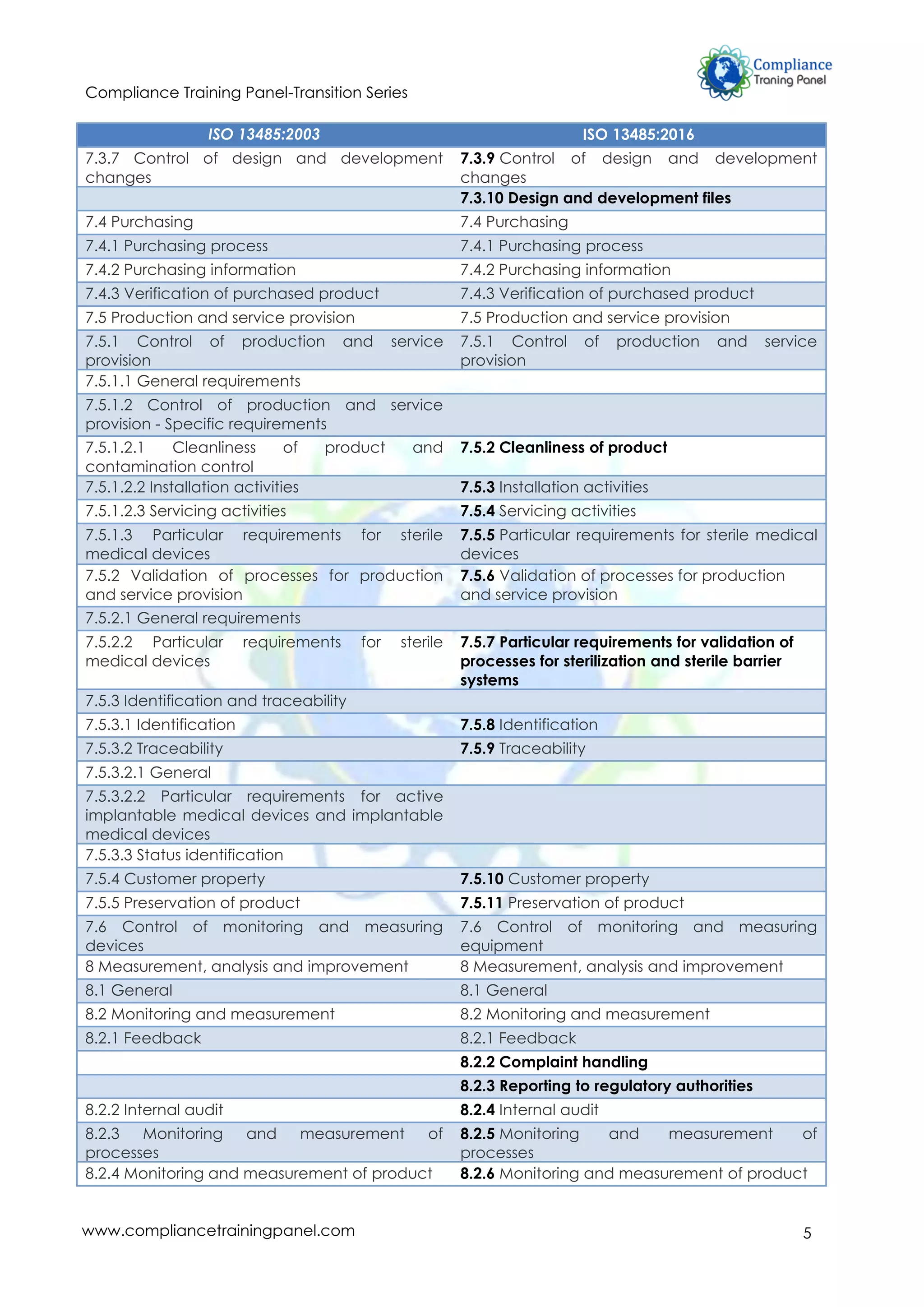
The document summarizes the changes from ISO 13485:2003 to ISO 13485:2016, highlighting a greater emphasis on risk management, compliance, and enhanced controls on outsourcing and documentation. It outlines key changes in requirements, such as the focus on medical device lifecycle, post-market surveillance, and assurance of safety and performance. New definitions and detailed clause-wise changes reflect the evolving complexities in the medical device field and aim to improve quality management systems.