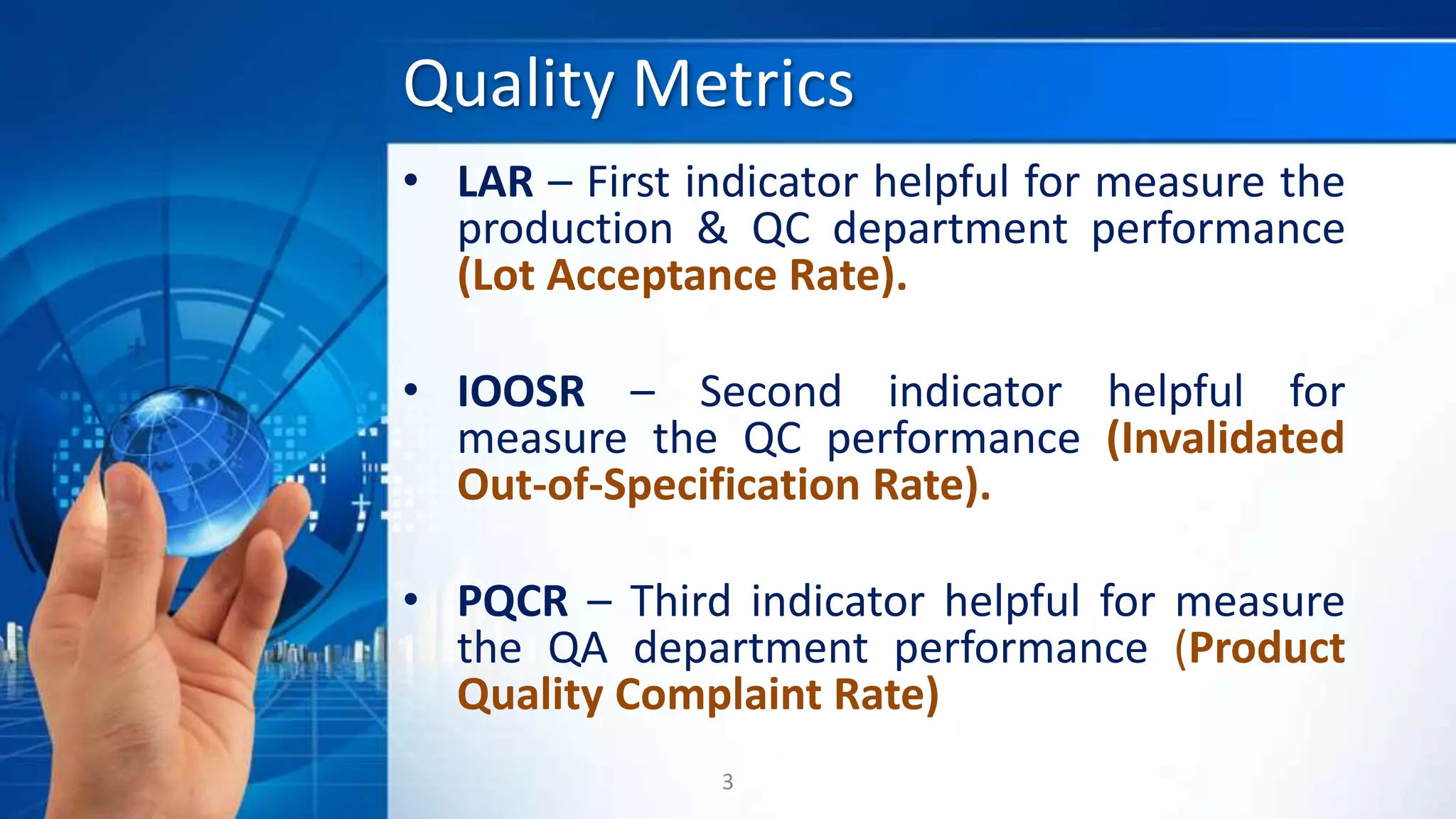
Quality metrics in the pharmaceutical industry are essential for monitoring processes, assessing departmental performance, and driving continuous improvement. Key metrics include Lot Acceptance Rate (LAR), Invalidated Out-of-Specification Rate (IOOSR), and Product Quality Complaint Rate (PQCR), each providing specific insights into manufacturing, quality control, and quality assurance. These metrics aim to ensure compliance and highlight areas needing justification when targets are not met.