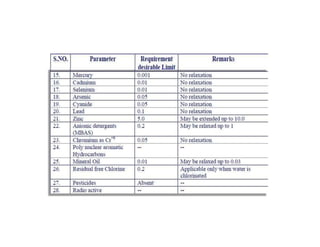
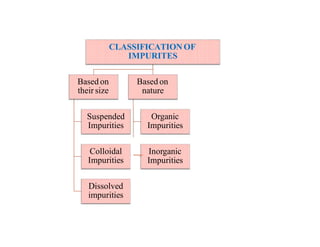
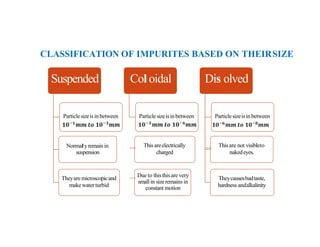
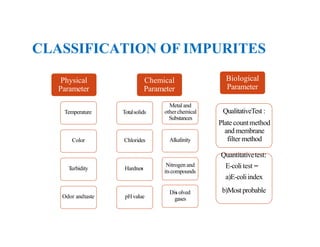
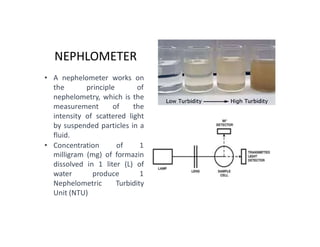
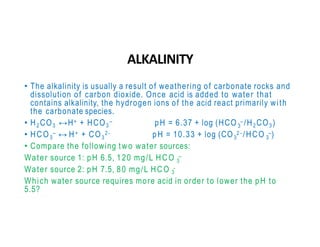
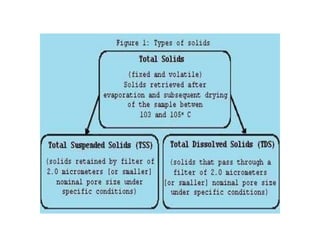
The document discusses various water quality parameters including water pollution, water quality standards, and important requirements of water for domestic use. It defines total solids as the total of all solids in a water sample, including total suspended solids, total dissolved solids, and volatile suspended solids. It also discusses how total solids are measured by weighing the solids present in a known water sample volume before and after drying to evaporate the water. The document provides classifications of impurities based on size and nature, and describes several common methods for analyzing water quality parameters.


















![Numerical
• [OH] ion concentration in water is 10^(-5.8) mole/L. Find the ph of
water?](https://image.slidesharecdn.com/waterqualityparameters-1-1-231025104058-47ea8735/85/WATER-QUALITY-PARAMETERS-1-1-pptx-24-320.jpg)







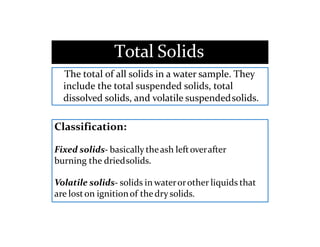







![➢The total solids concentration isequal to
the difference between the weight of the
beaker with the residue and the weight
of the beaker withoutit.
Total Solids (mg/L) :
Total solids(TS) = [(TSA – TSB)] /V
TSA = Weightof dried residue + dish in milligrams
TSB = Weight of dish inmilligrams
V= Volume of Sample(mL)](https://image.slidesharecdn.com/waterqualityparameters-1-1-231025104058-47ea8735/85/WATER-QUALITY-PARAMETERS-1-1-pptx-42-320.jpg)
