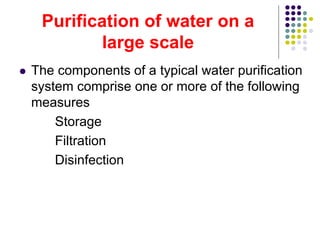
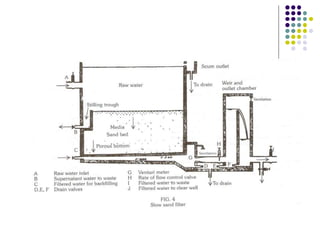
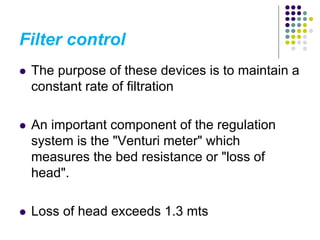
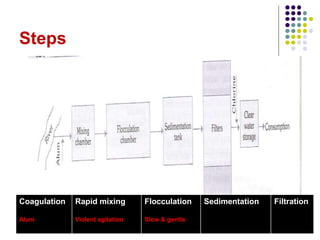
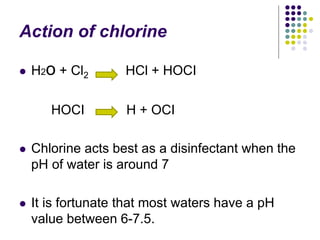
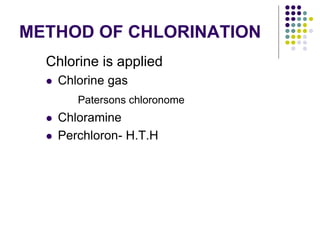
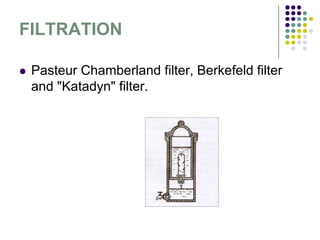
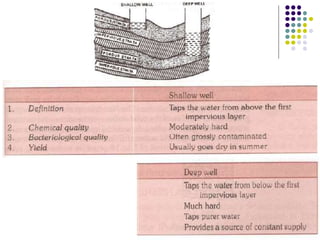
The document discusses environmental health and sanitation. It defines environment and discusses various physical, biological, and social environmental factors. Poor sanitation and pollution of air, water, and soil are linked to ill health. Maintaining a healthy environment requires a multi-disciplinary approach. The document focuses on water specifically, outlining different water sources, related health issues, and purification methods. It emphasizes the importance of access to safe drinking water and discusses large-scale water treatment involving storage, filtration, and chlorine disinfection.













































![Water pollution law
Parliament in 1974 passed water [prevention
and control of pollution] act
It provides for the constitution of Central and
State Water boards and Joint Water Boards
capable with wide powers for controlling
pollution.](https://image.slidesharecdn.com/puriwater-220427114007/85/ENVIRONMENT-AND-HEALTH-ppt-1-ppt-48-320.jpg)