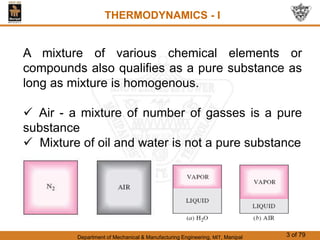
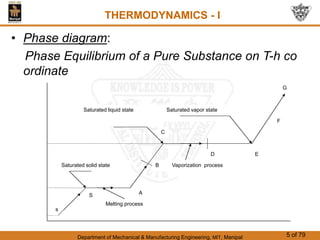
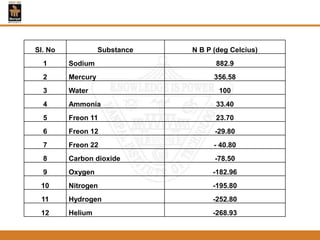
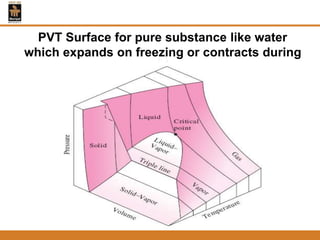
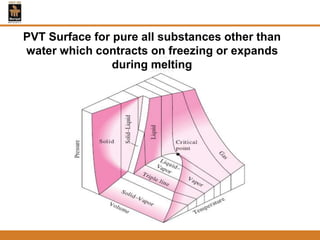
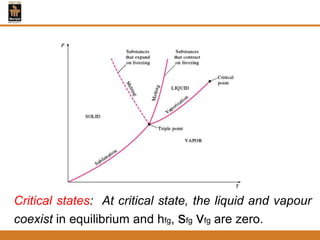
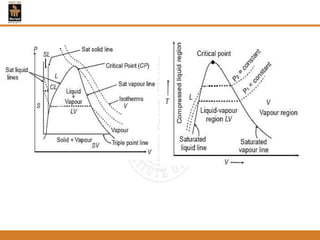
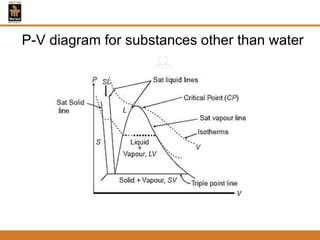
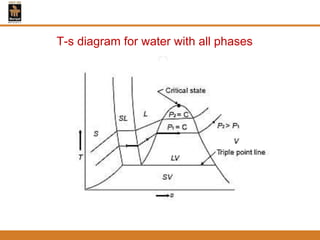
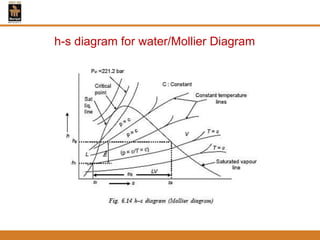
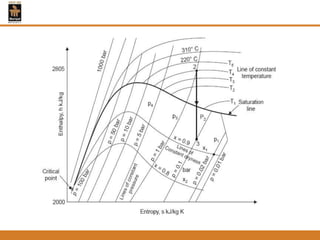
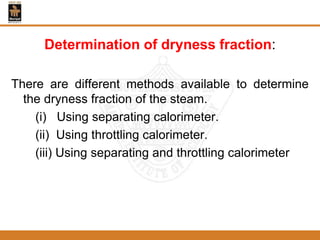
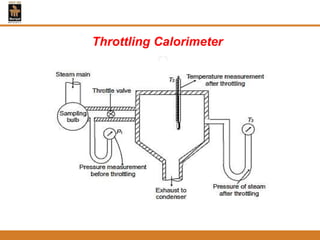
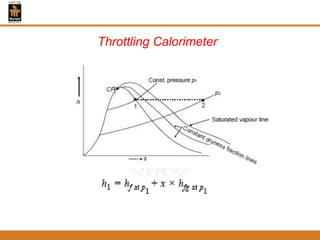
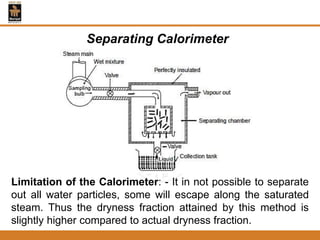
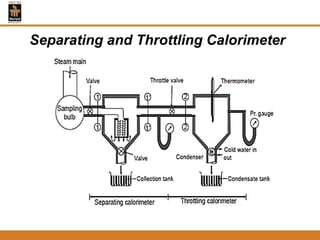
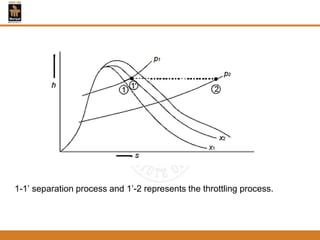
The document discusses pure substances and their properties. It defines a pure substance as one that is homogeneous and has a constant chemical composition across all phases. A pure substance can exist as a solid, liquid or gas, and its chemical makeup remains the same in each phase. The document also describes phase diagrams and equations of state for pure substances, and methods for determining the dryness fraction of steam using calorimeters.