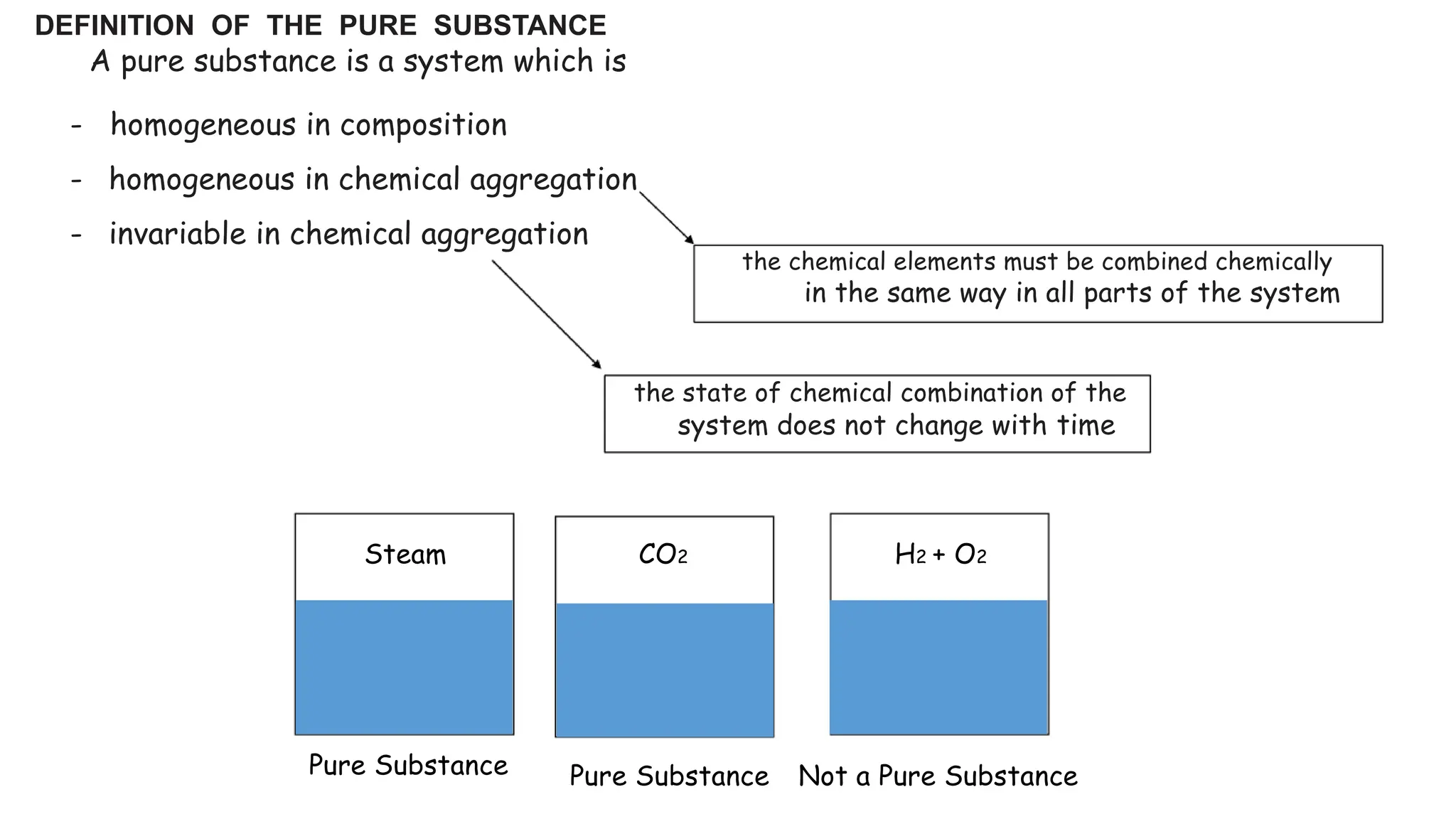
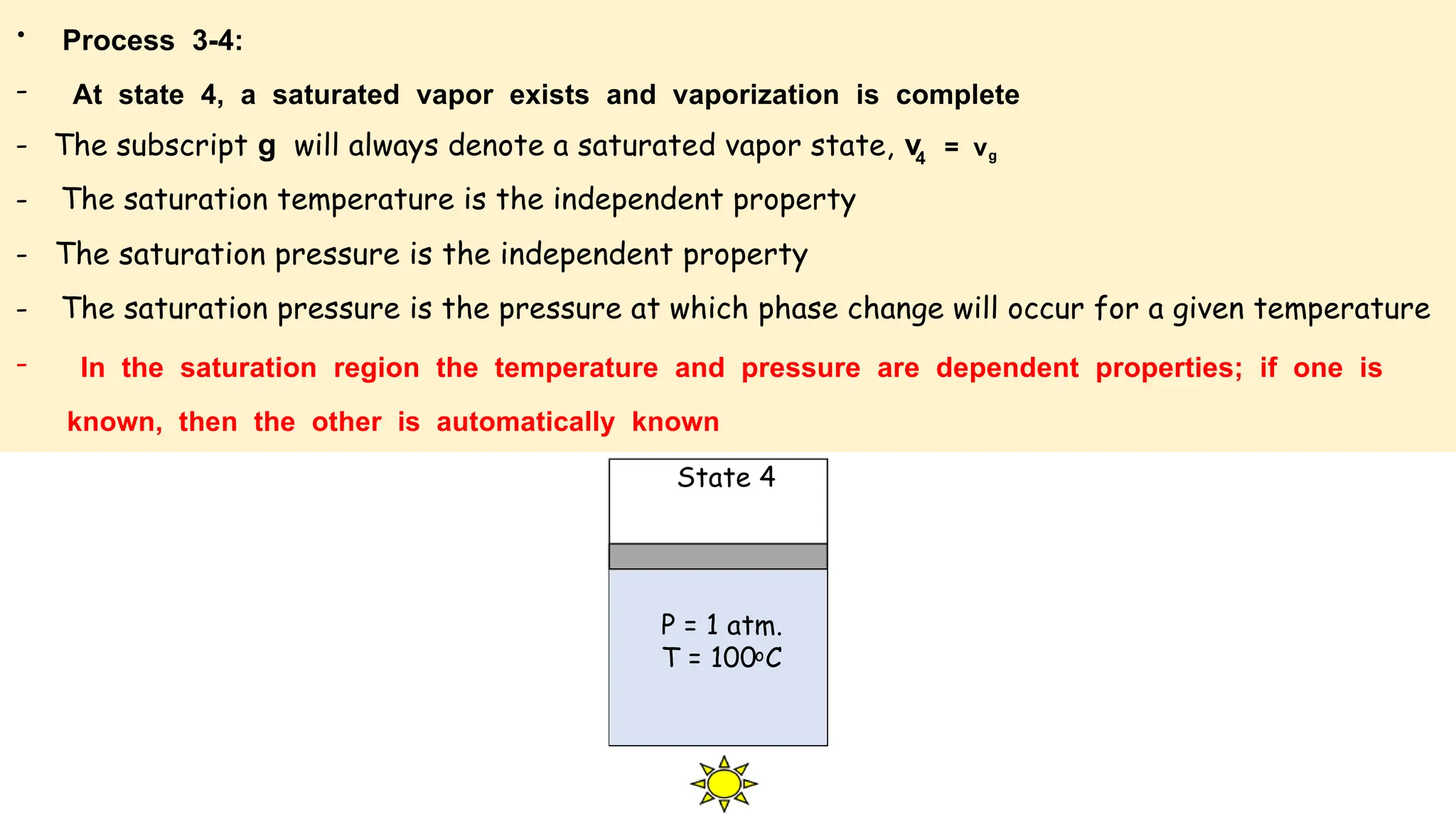
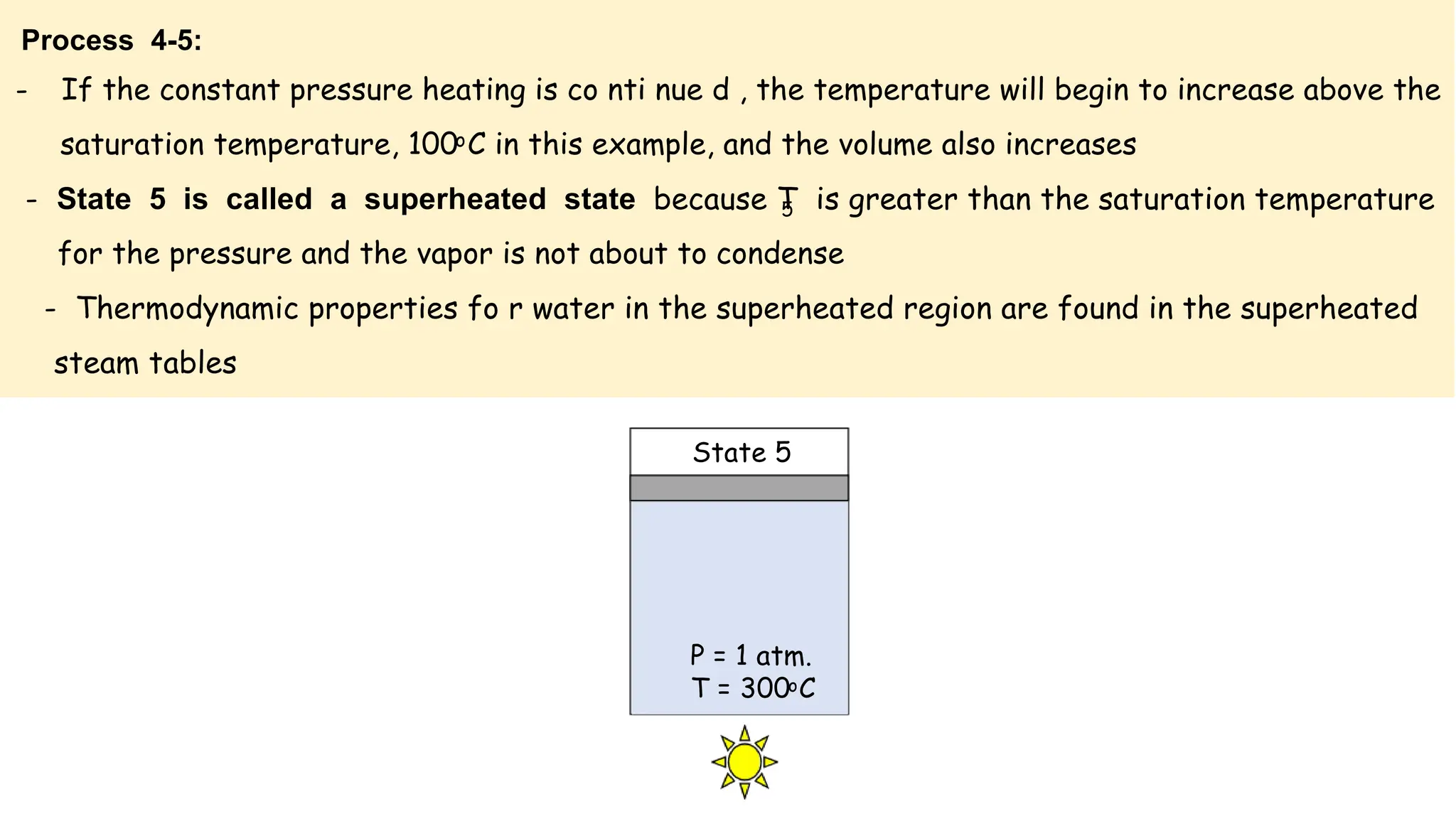
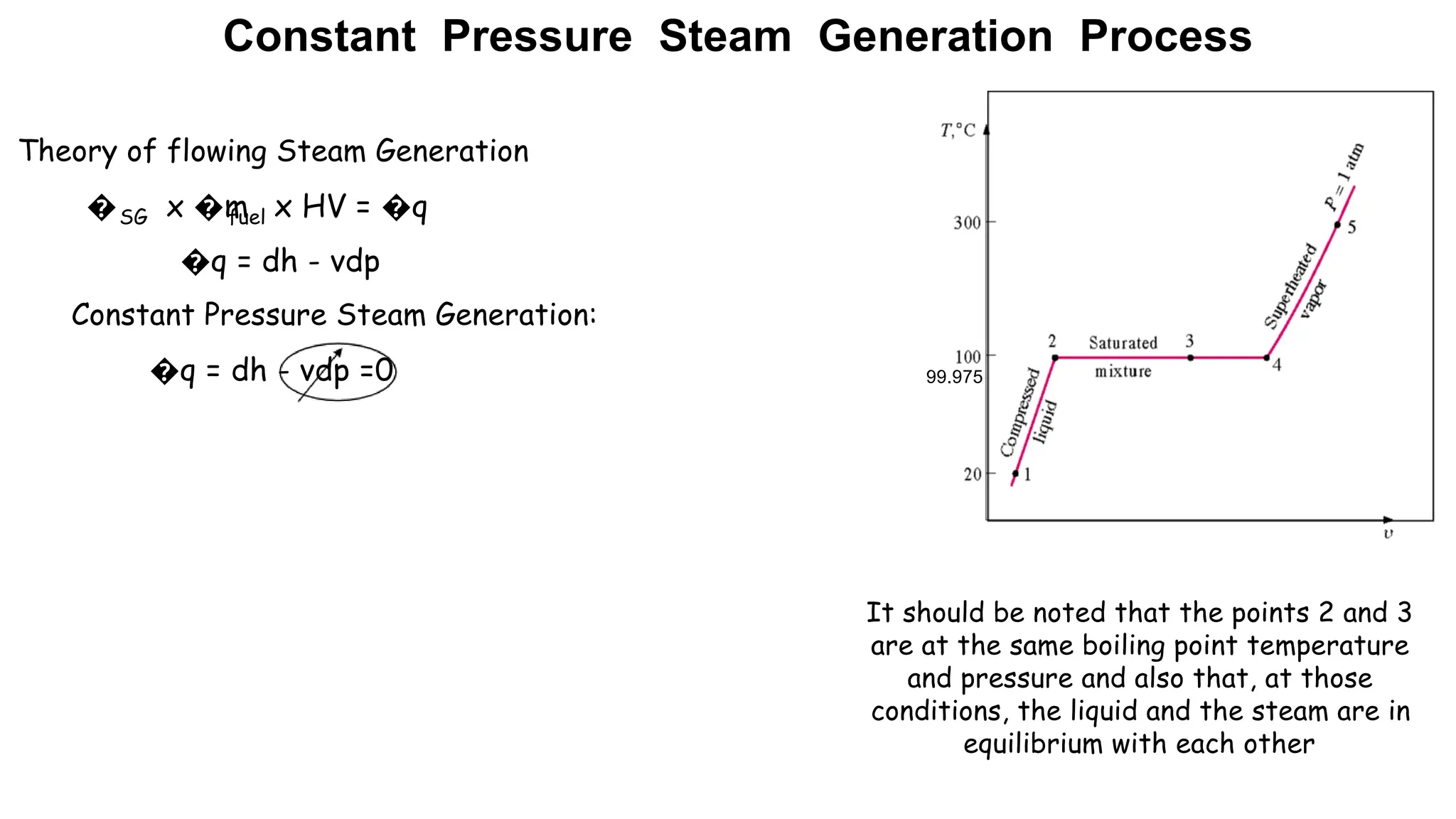
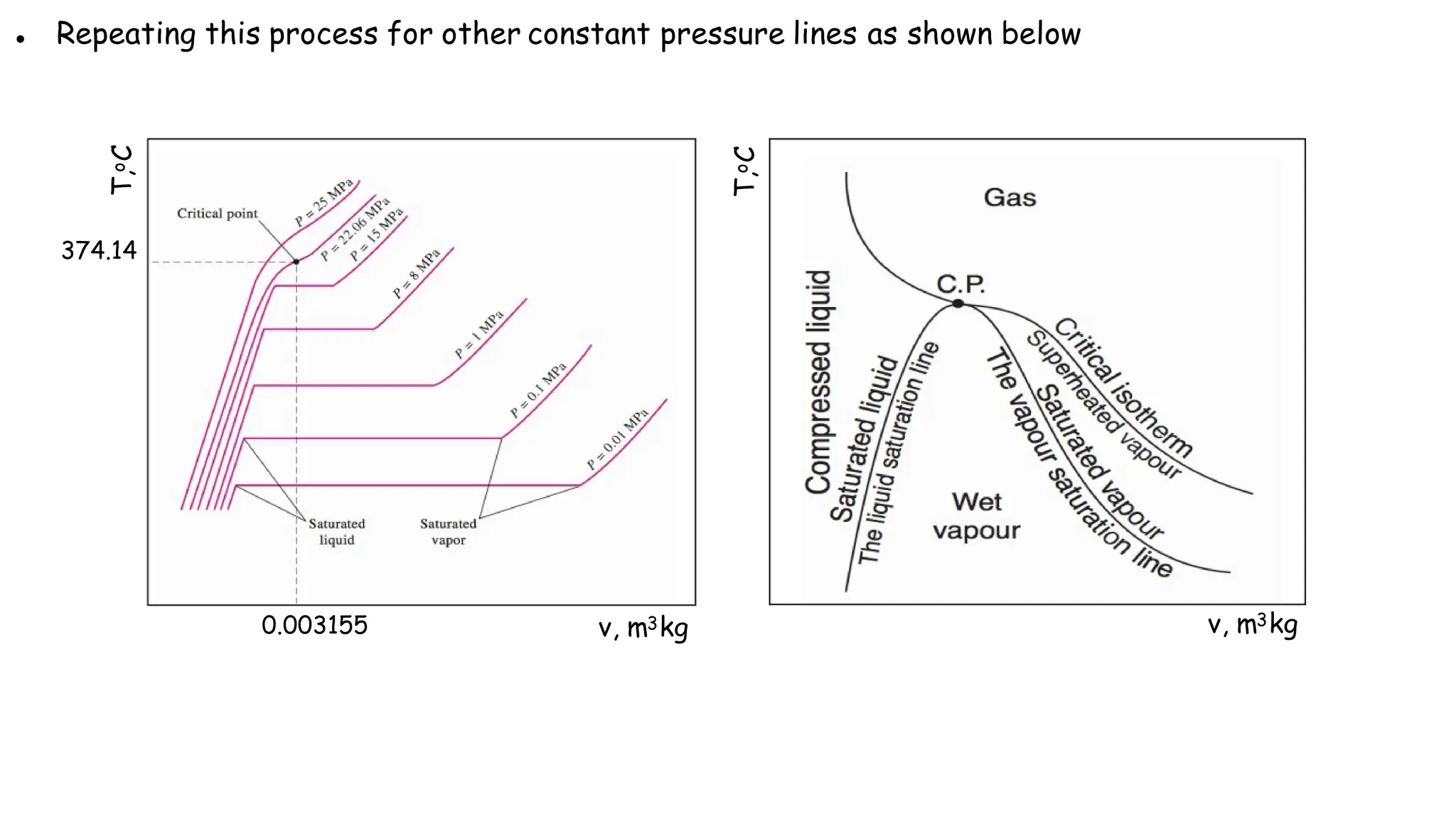
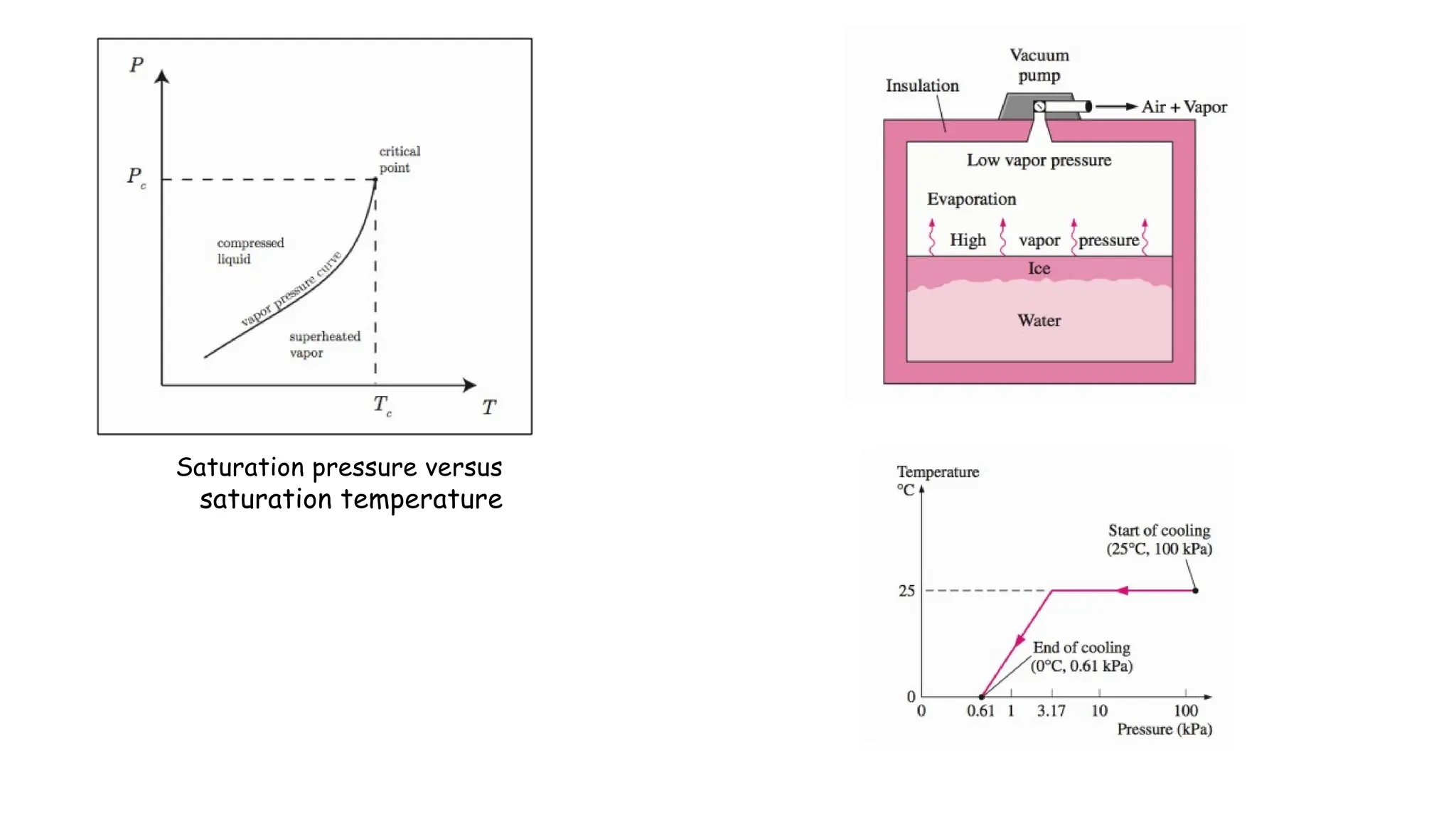
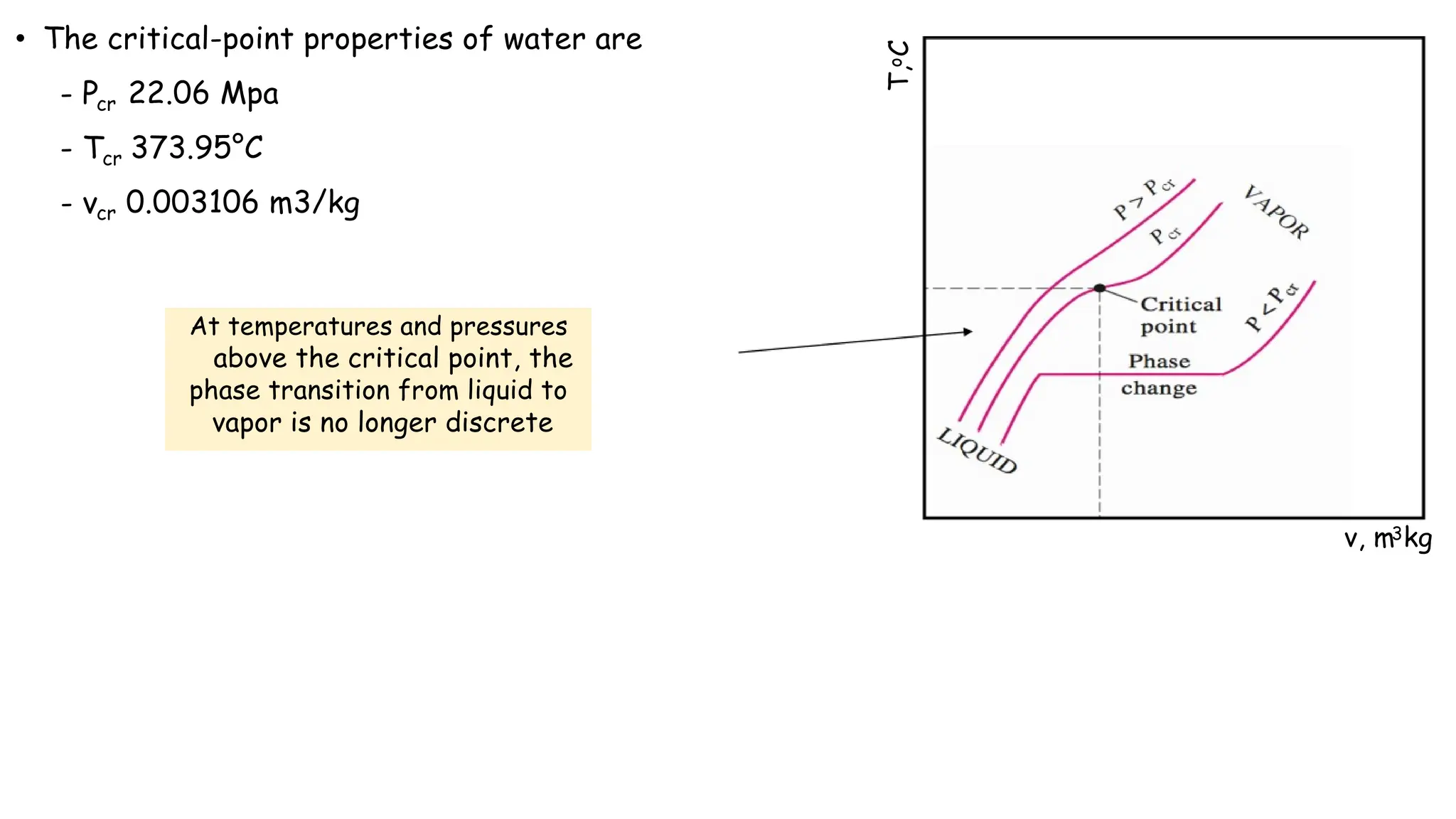
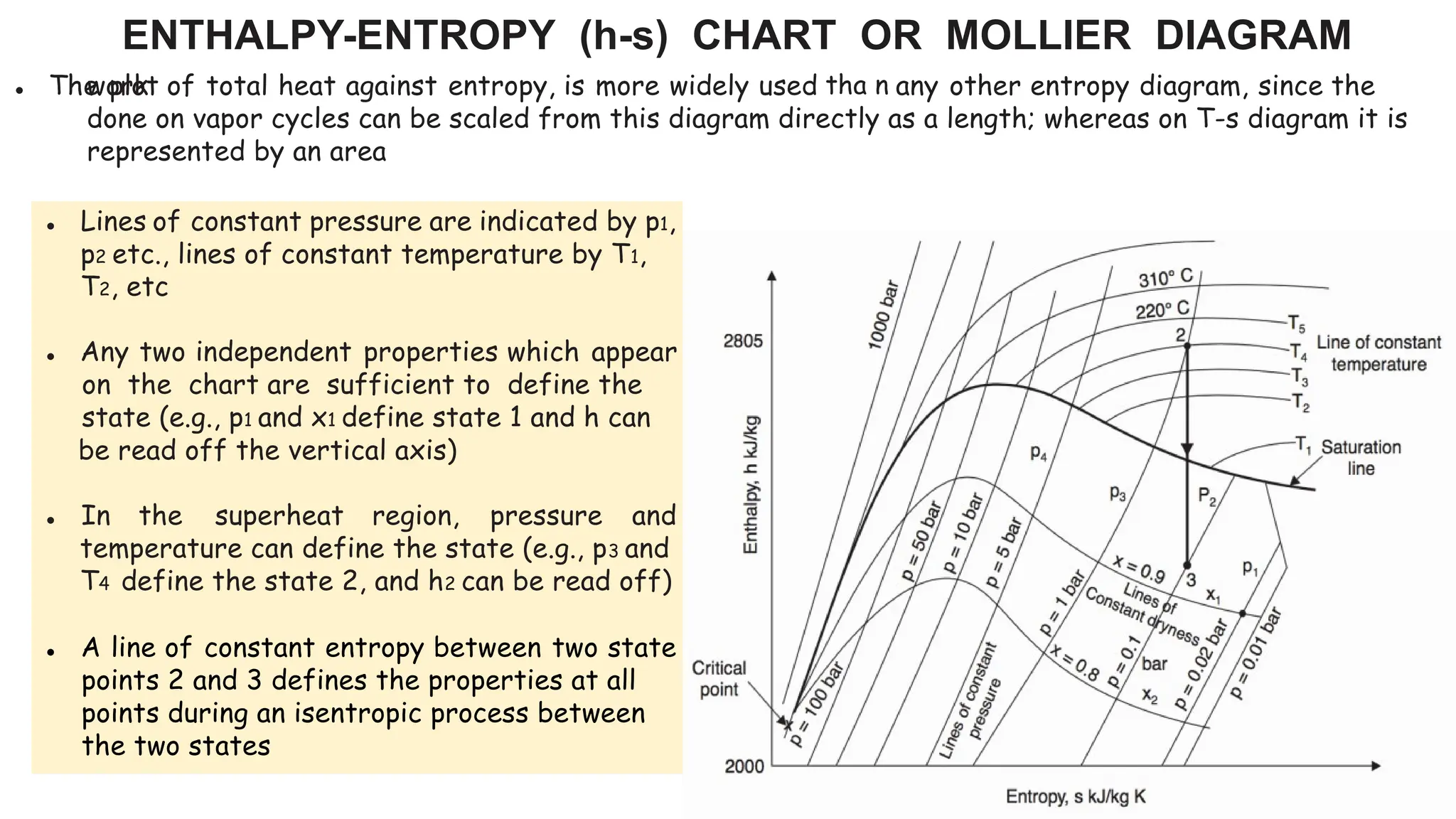
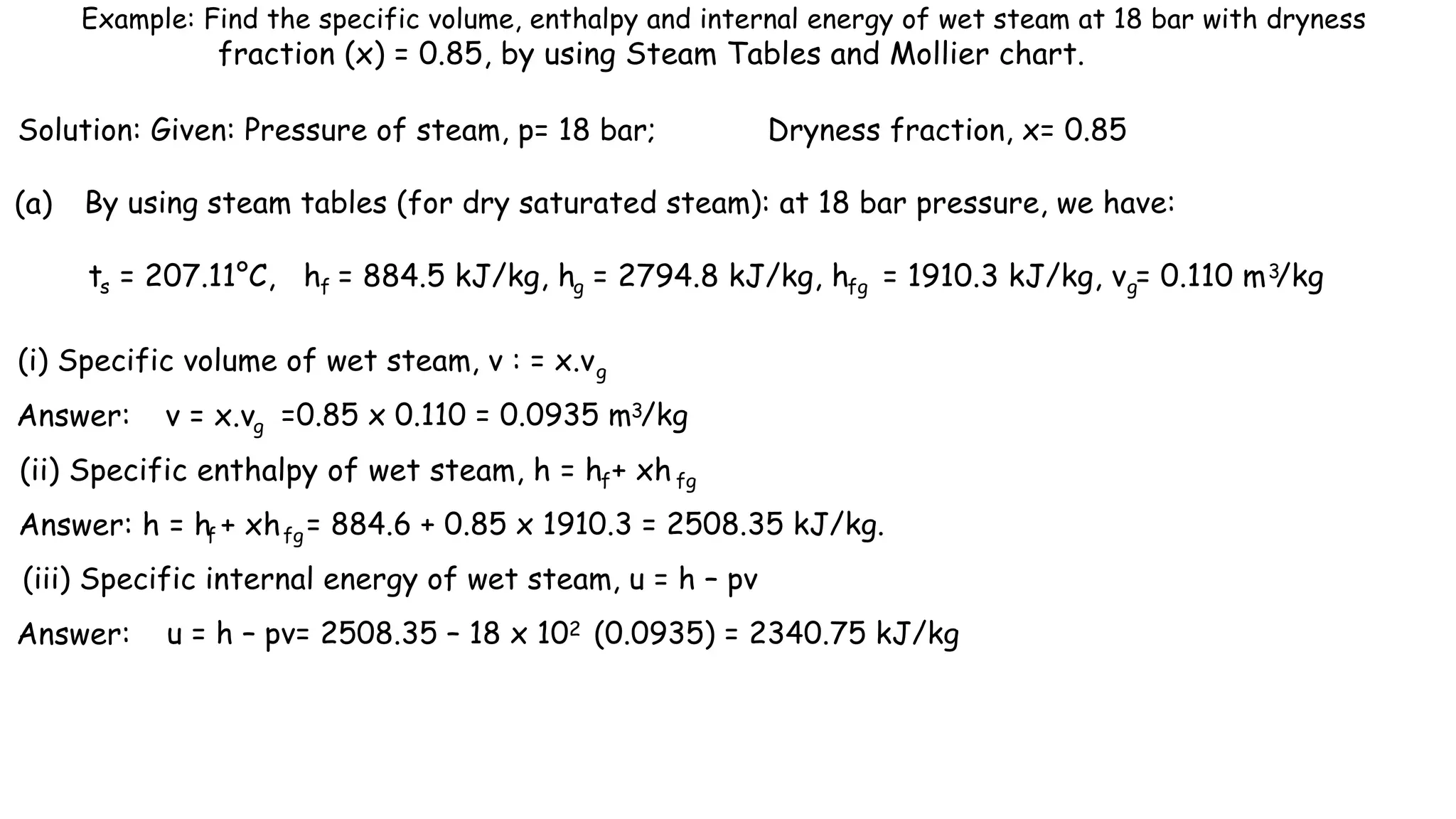
The document outlines the properties and behavior of pure substances, particularly focusing on phase changes, thermodynamic states, and the definitions of terms like saturated vapor, superheated steam, and wet steam. It details the heating process of liquid water under constant pressure, explaining phenomena such as boiling and vaporization, and provides information on the critical point and the Mollier diagram for determining steam properties. It also emphasizes the importance of parameters like dryness fraction in measuring steam quality and enthalpy in steam systems.