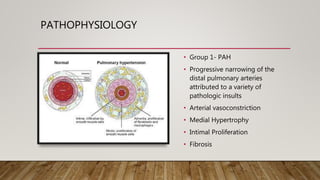
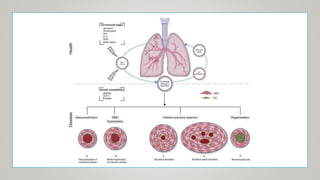
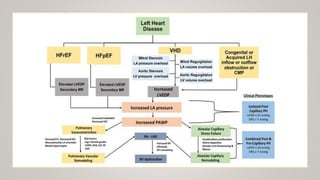
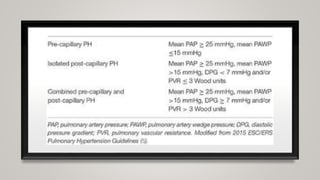
This document discusses the pathophysiology and diagnosis of pulmonary hypertension. It begins by defining pulmonary hypertension and describing the clinical classification system. It then covers the pathophysiology of the different groups, explaining how increases in pulmonary vascular resistance can lead to elevated pulmonary artery pressures. The diagnosis section notes that symptoms are often non-specific and outlines the steps of evaluation, including imaging, echocardiography, pulmonary function tests and right heart catheterization.