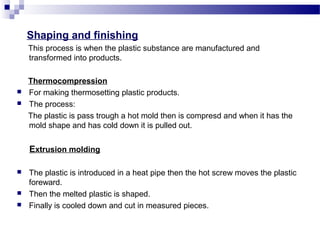
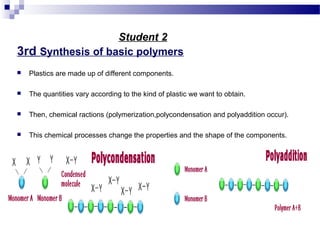
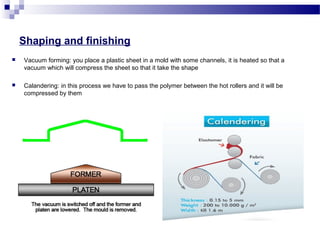
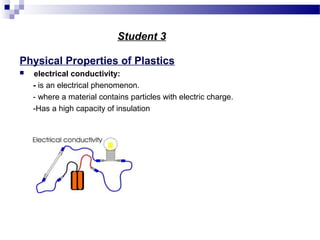
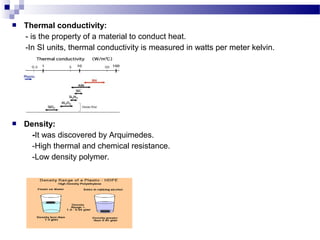
This document discusses the process of plastics production from four student perspectives. Student 1 describes the sourcing of raw materials from plants, animals, and minerals, and the initial modification process. Student 2 then discusses the synthesis of monomers, addition of additives, and various shaping techniques like injection molding. Student 3 outlines some physical properties of plastics like electrical conductivity and thermal expansion. Finally, Student 4 categorizes plastics into thermosets, thermoplastics and elastomers and their characteristics and applications.