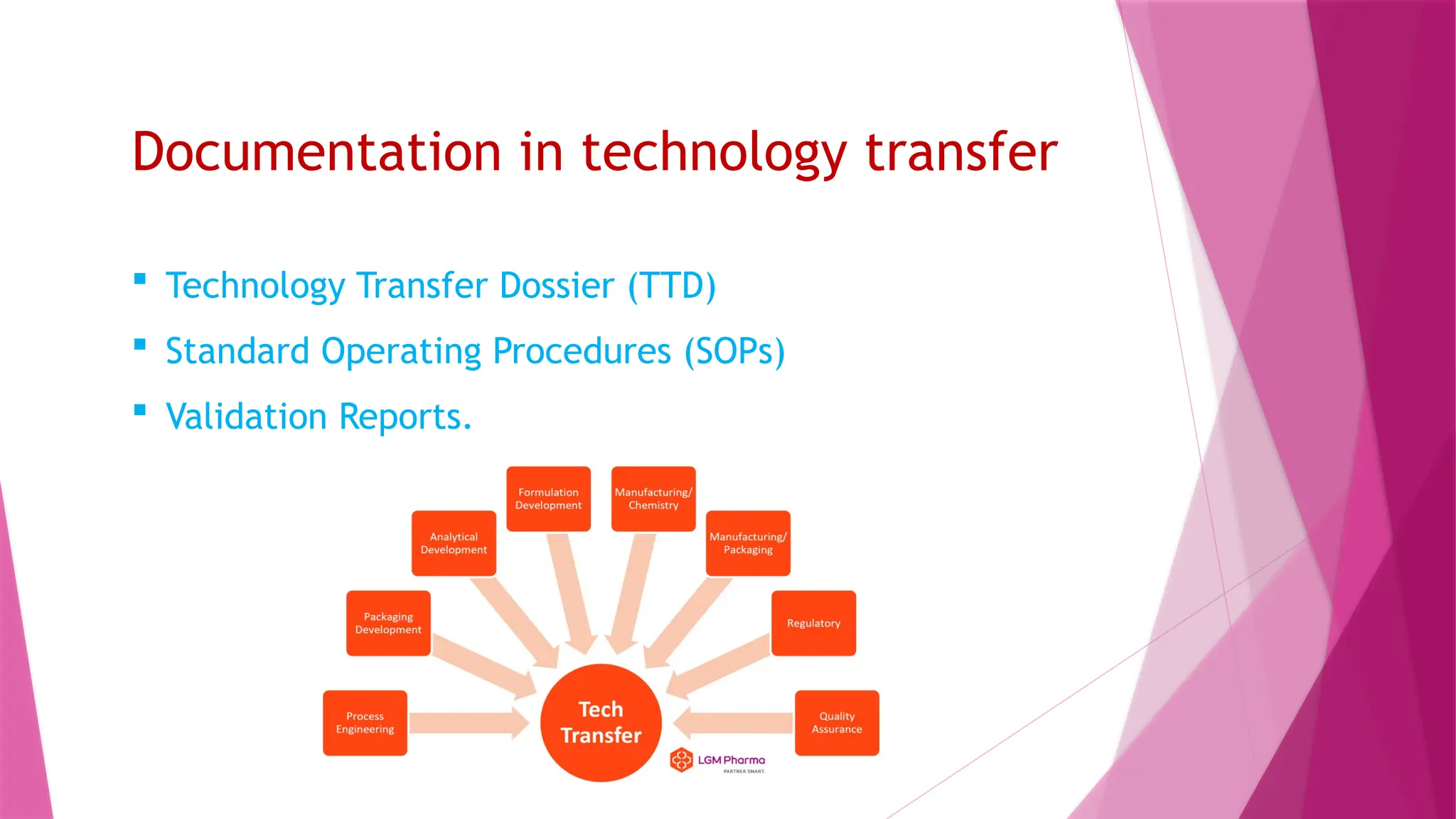
The document outlines the process of technology transfer in the pharmaceutical industry, emphasizing its importance for improving efficiency, reducing costs, and enhancing product quality. It details definitions, types, stages, benefits, regulatory considerations, and challenges associated with technology transfer. The conclusion highlights the critical role of understanding technology transfer for successful commercialization in industrial pharmacy.




![Types of
Technology Transfer
Intra-organizational transfer [within company]
Inter-organizational transfer [between company]
International transfer [across countries]](https://image.slidesharecdn.com/technologytransferpptip88-241211064622-ac5b9fe8/75/TECHNOLOGY-TRANSFER-Definitions-Importance-process-and-Applicationsip-88-pptx-5-2048.jpg)