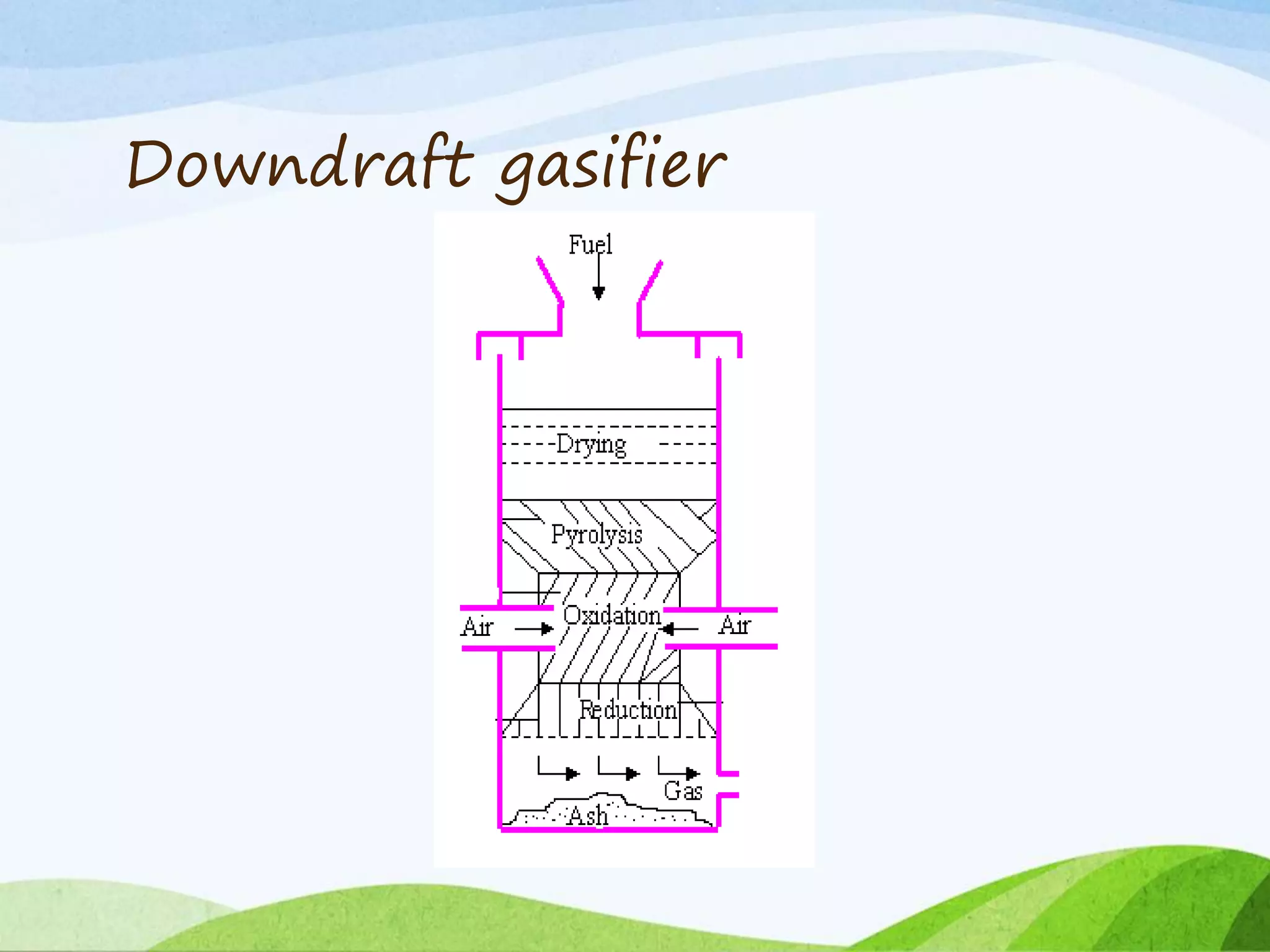
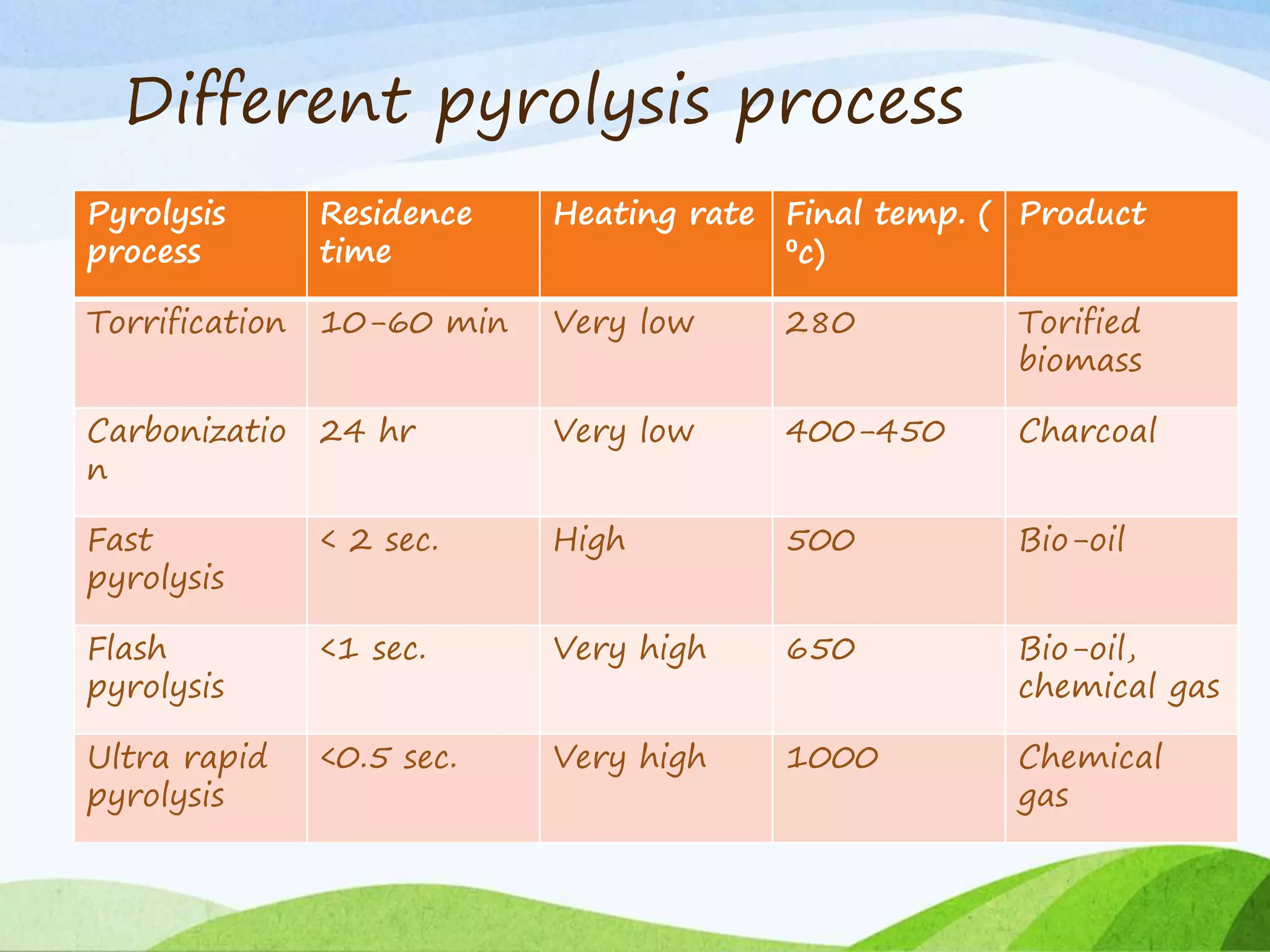
The document presents an overview of producer gas production from biomass through gasification, detailing its constituents, applications, and types of gasifiers. It explains the gasification process, including drying, pyrolysis, oxidation, and reduction reactions, which yield producer gas rich in useful components for energy generation. Different gasifier types, their advantages and disadvantages, and the efficiency of gasification compared to direct combustion are also discussed.