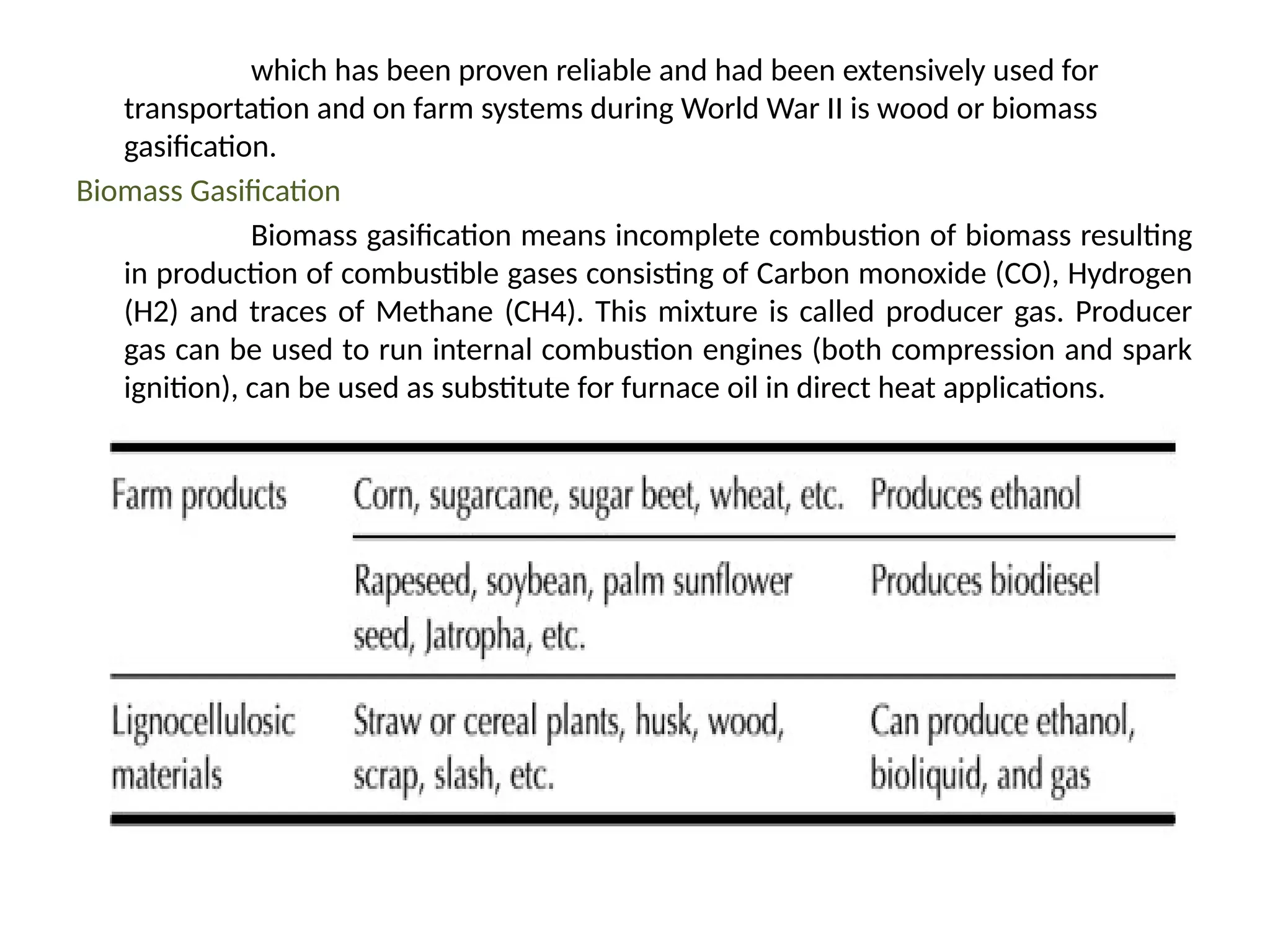
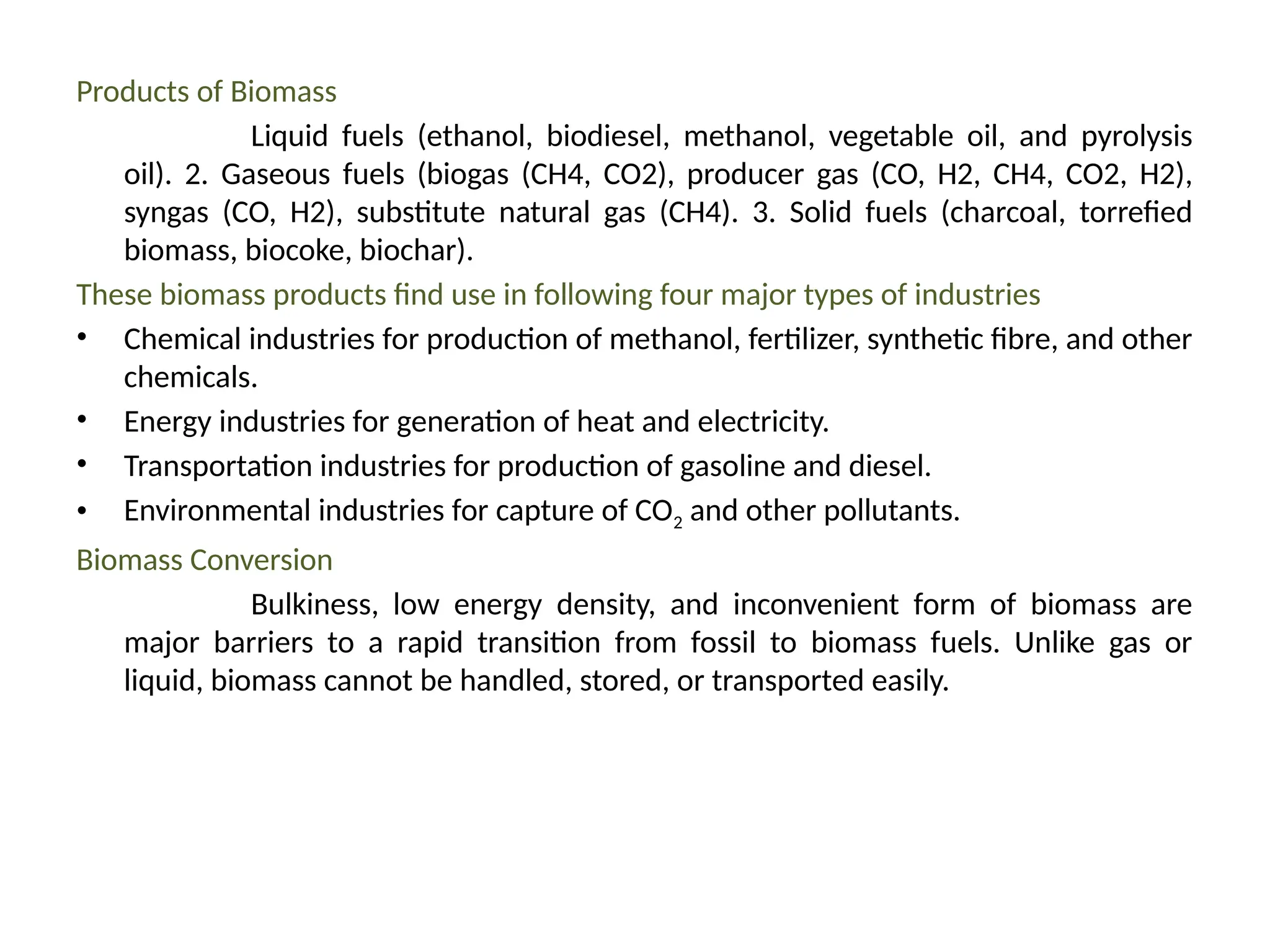
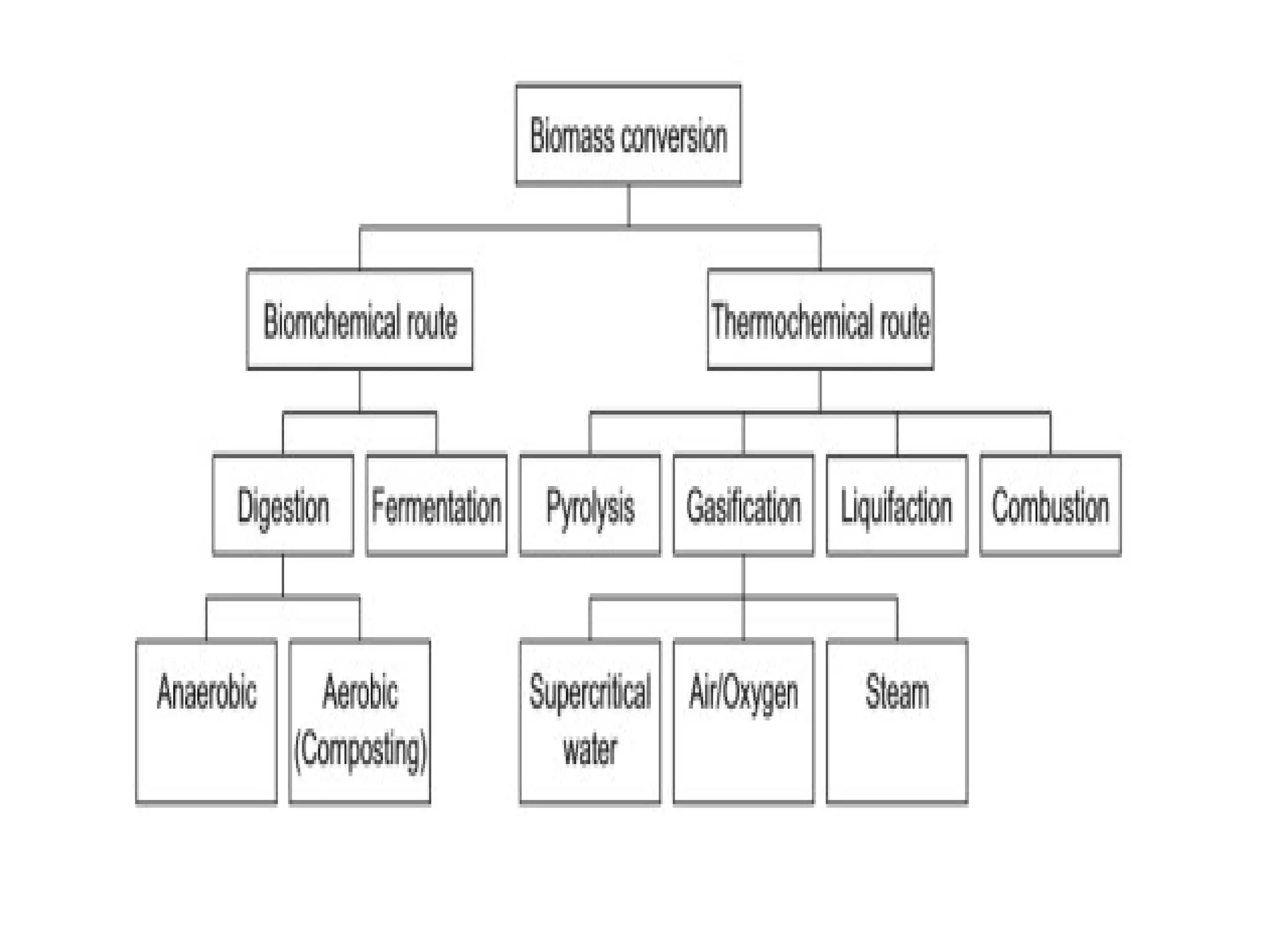
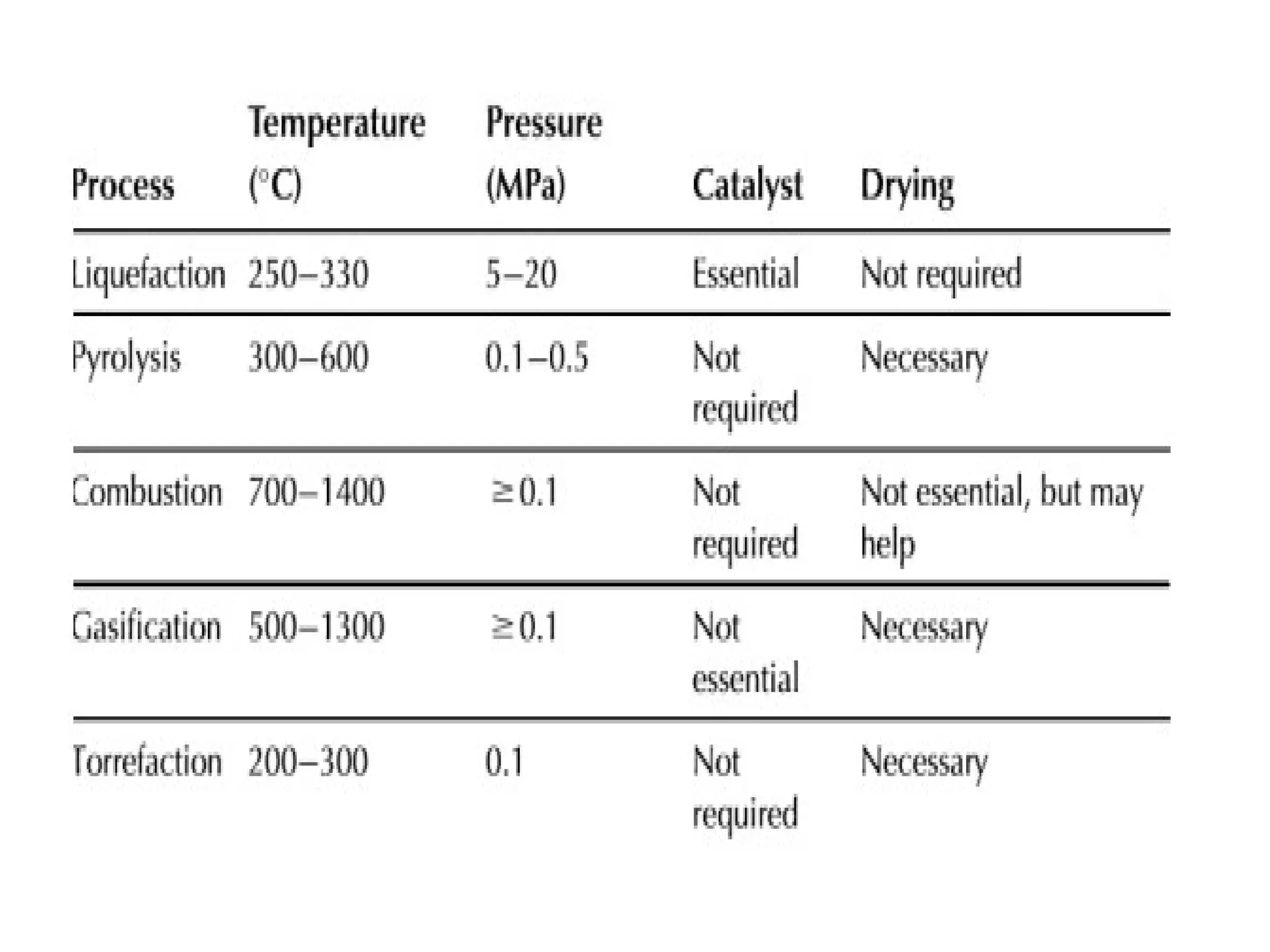
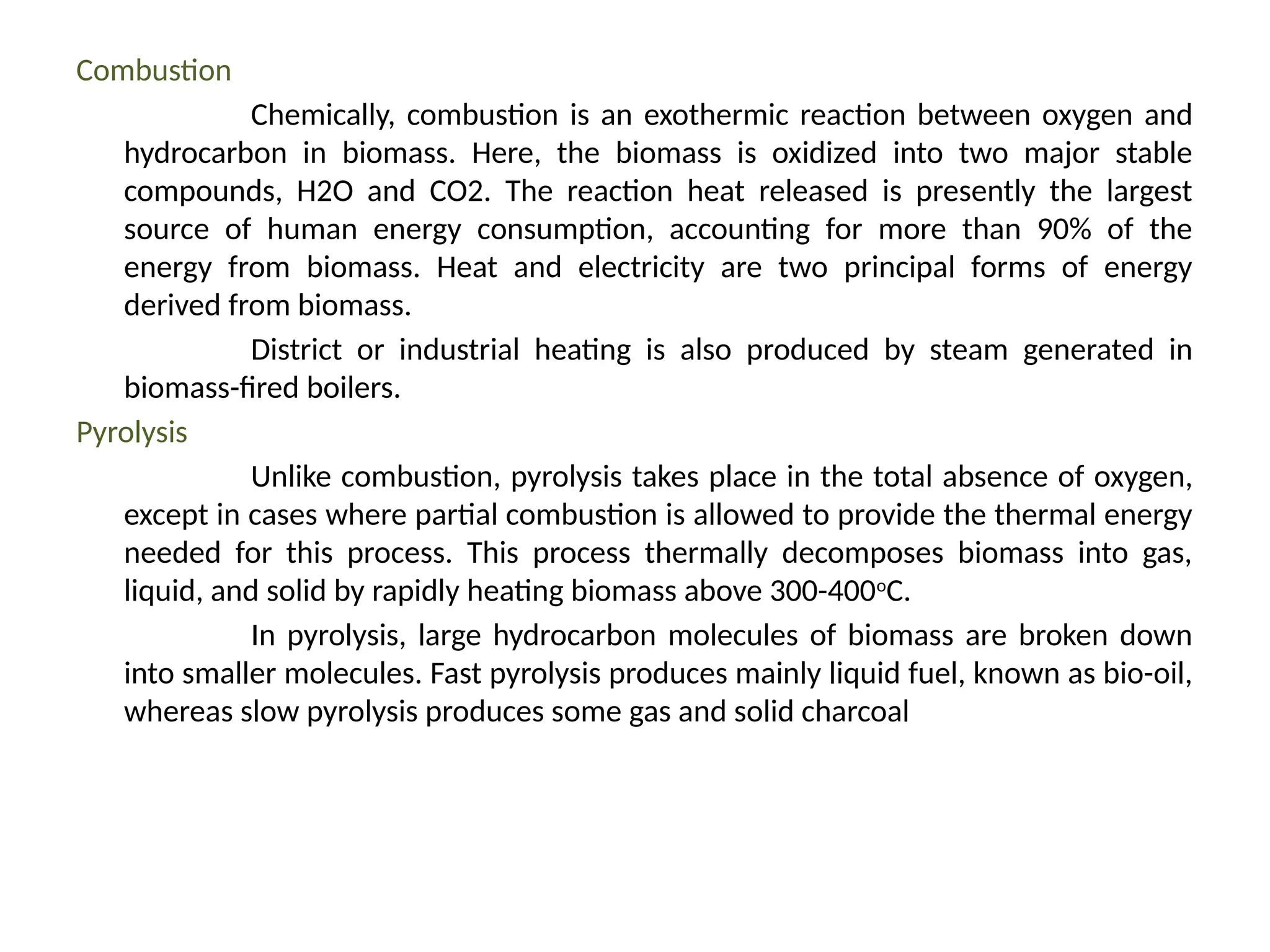
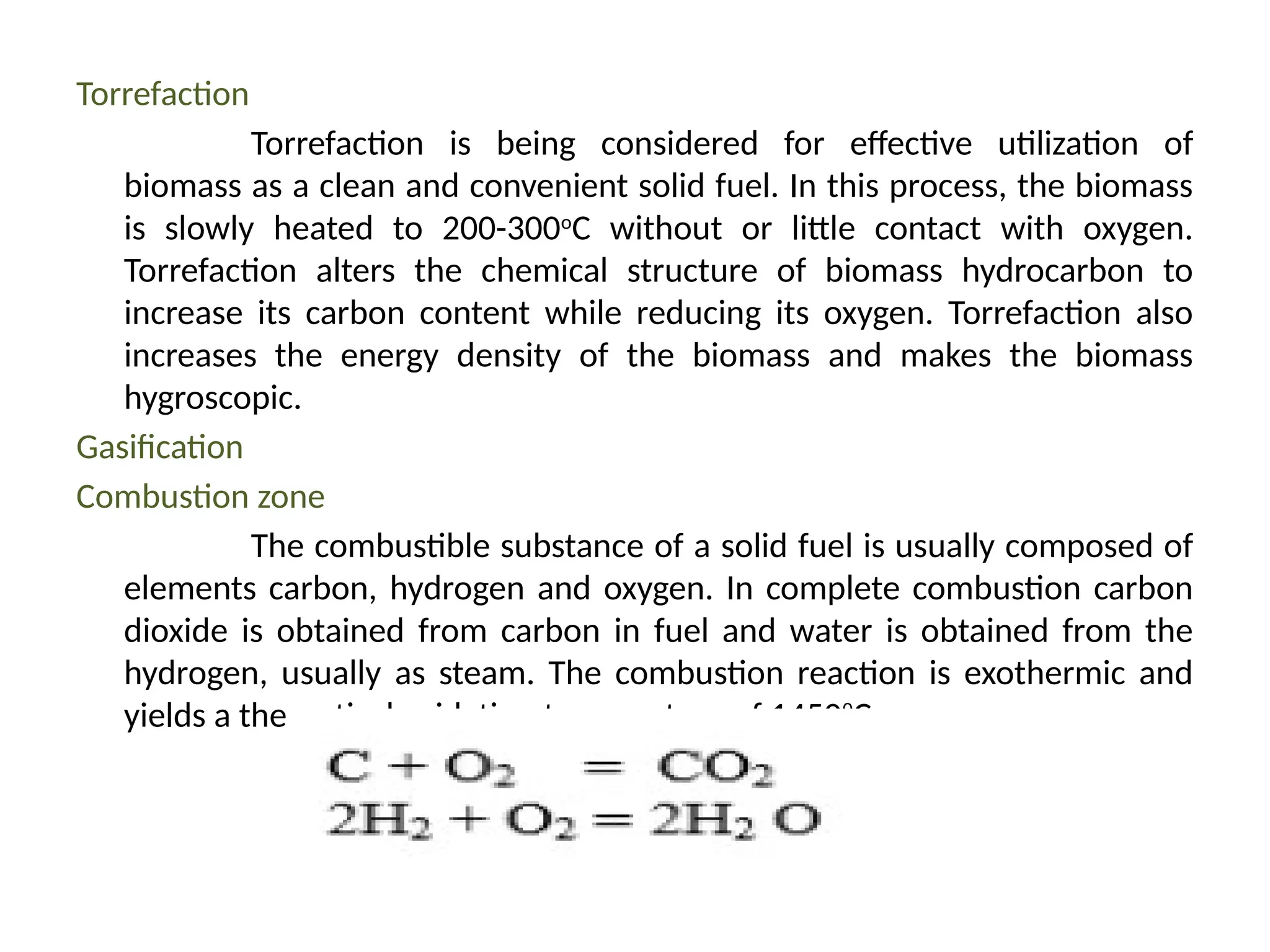
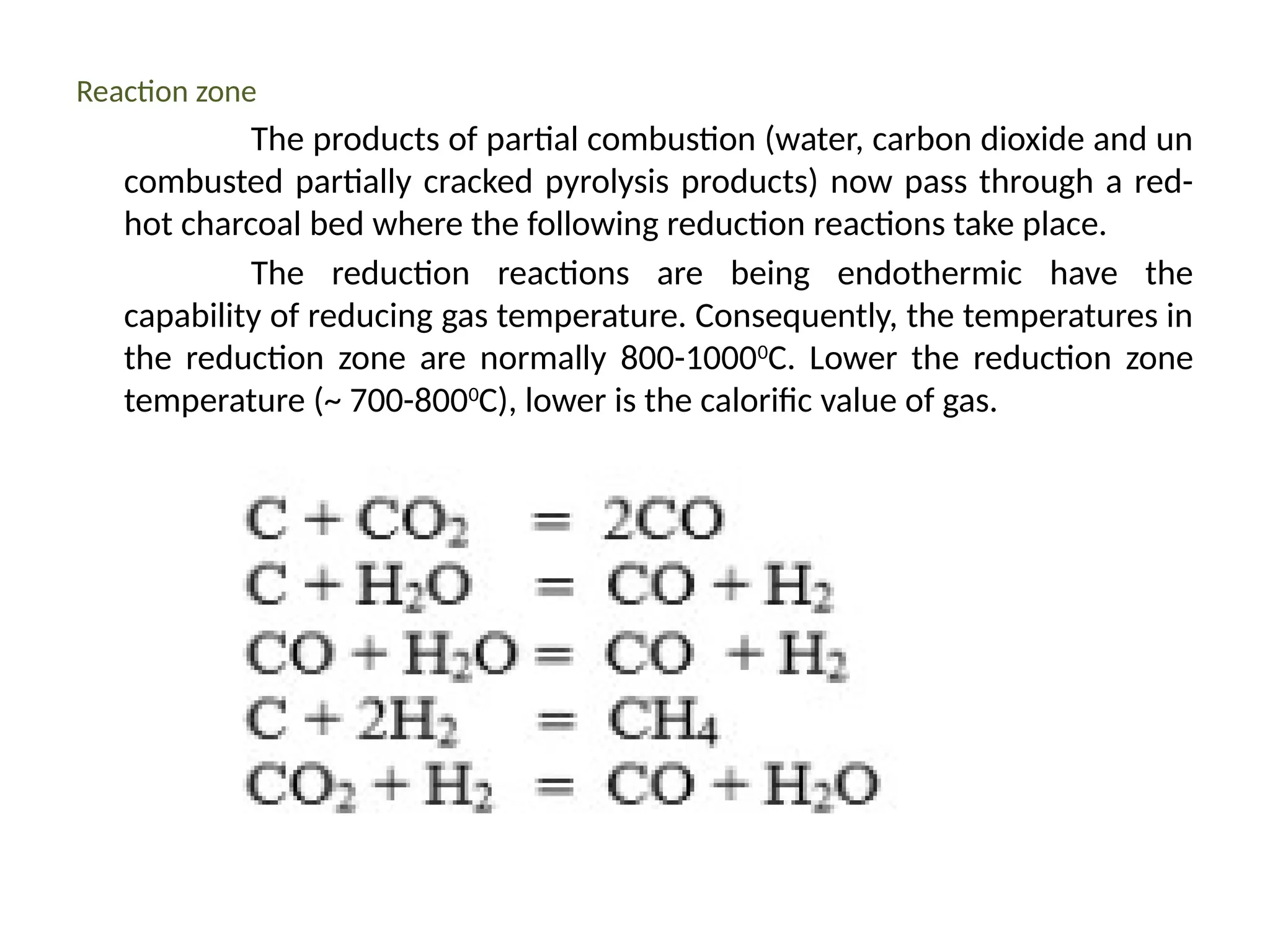
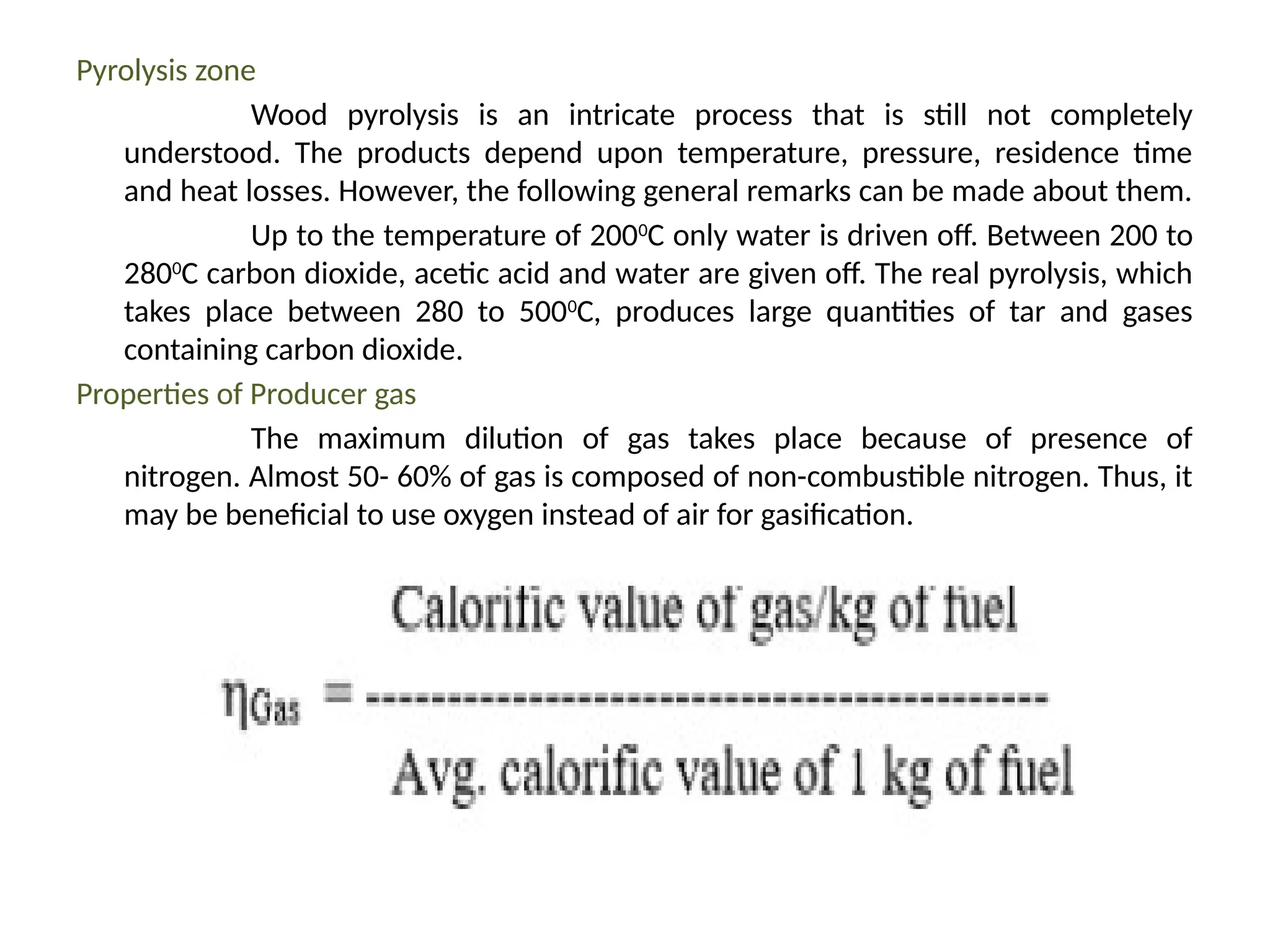
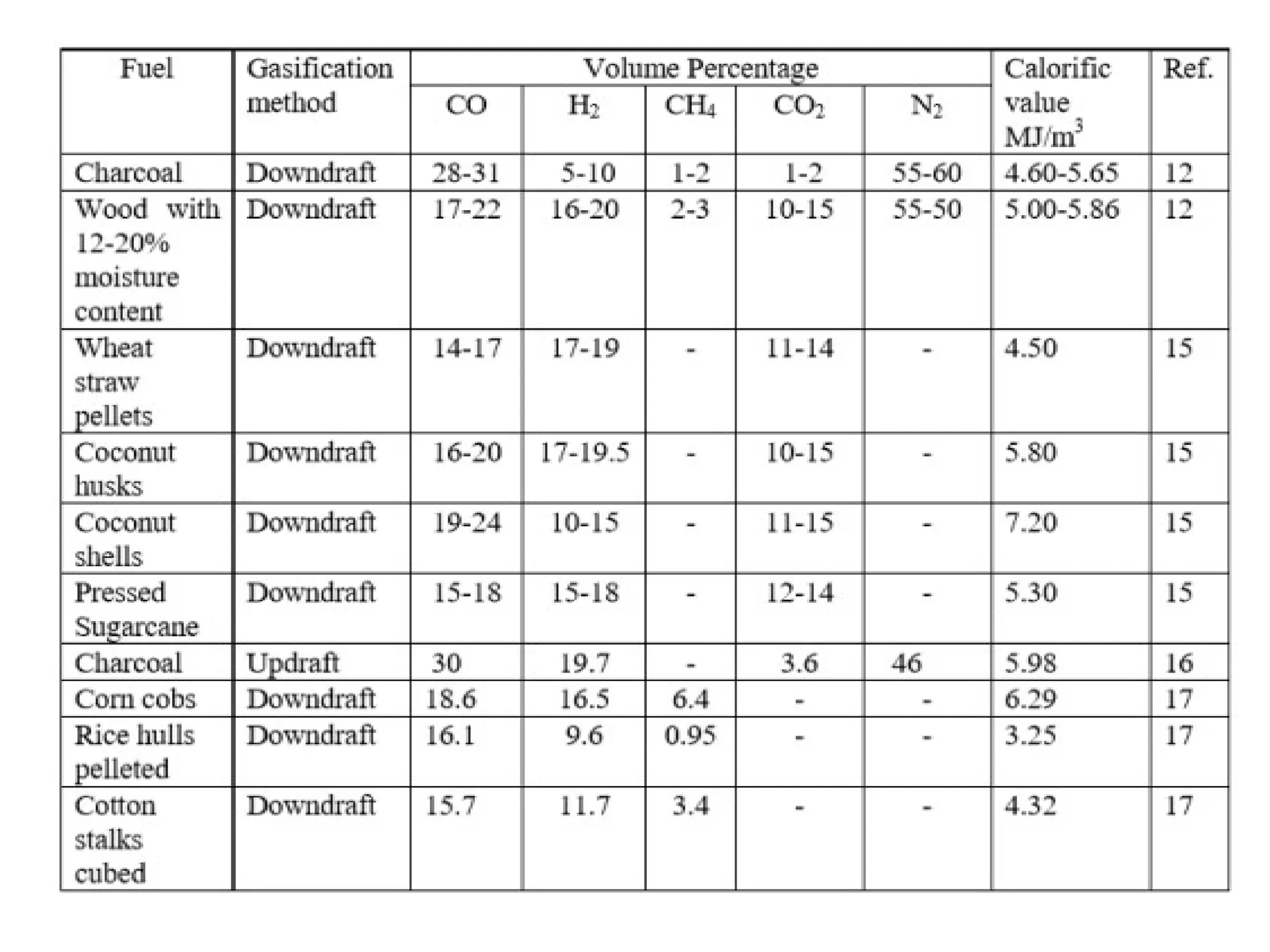
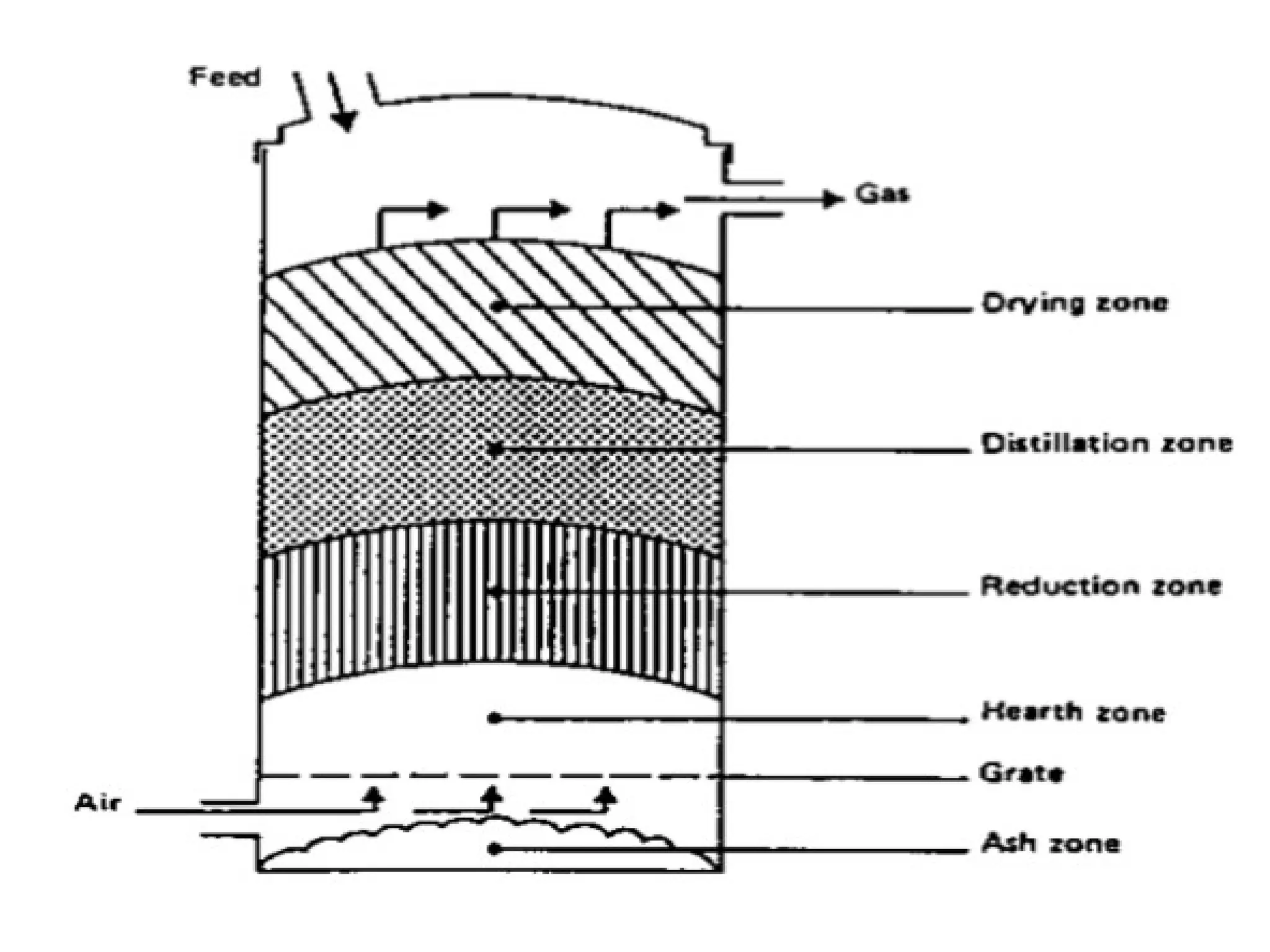
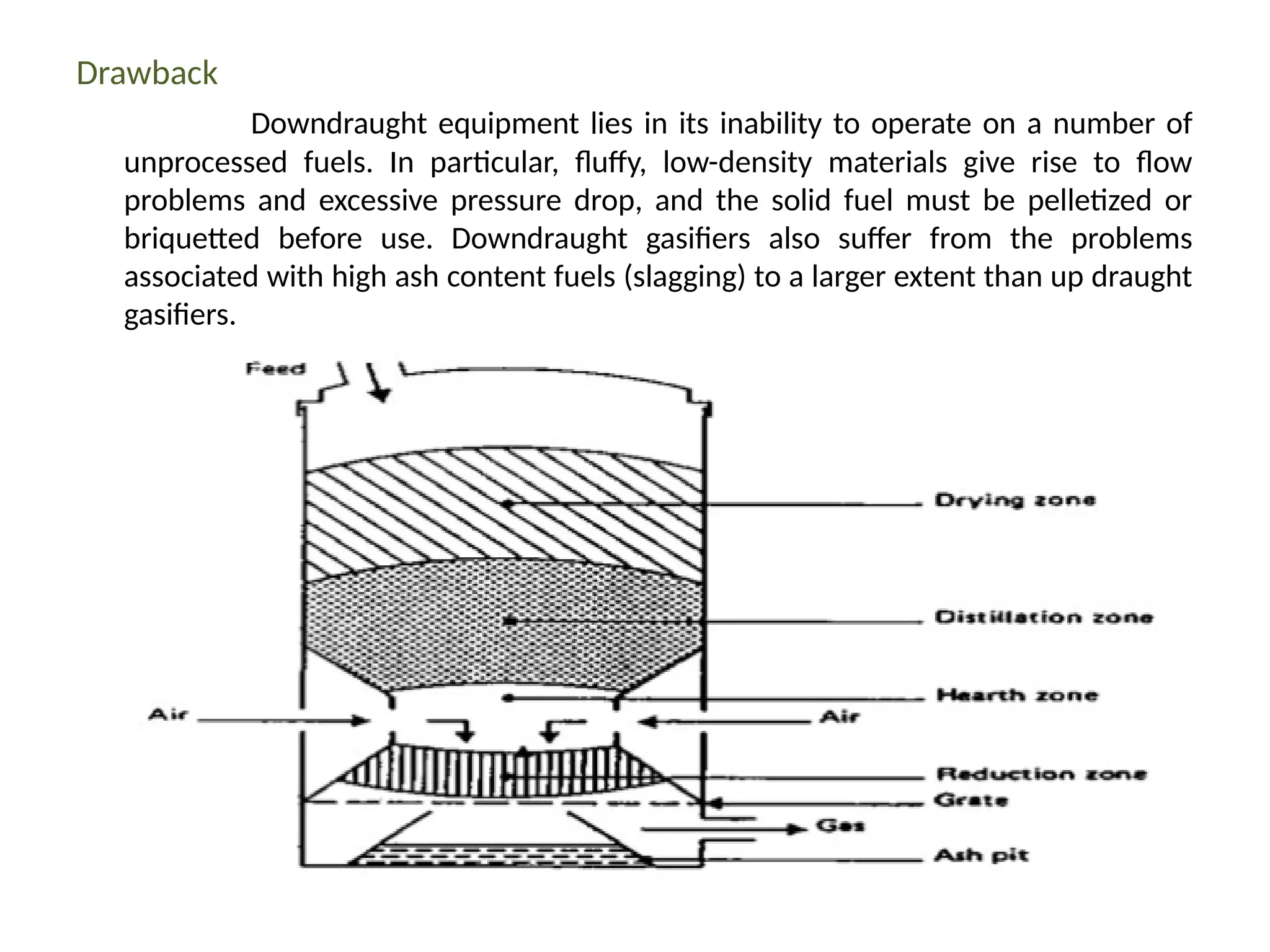
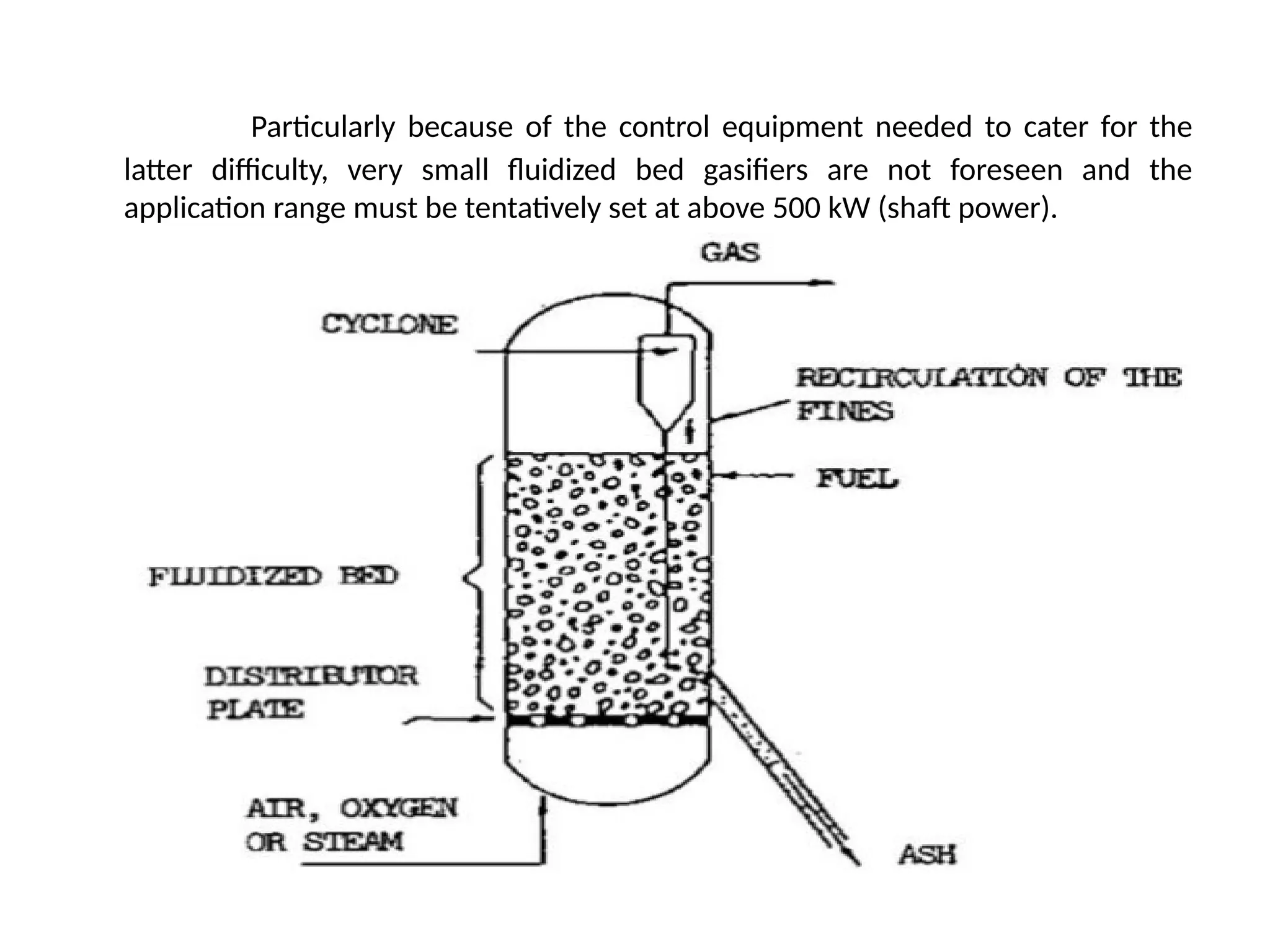
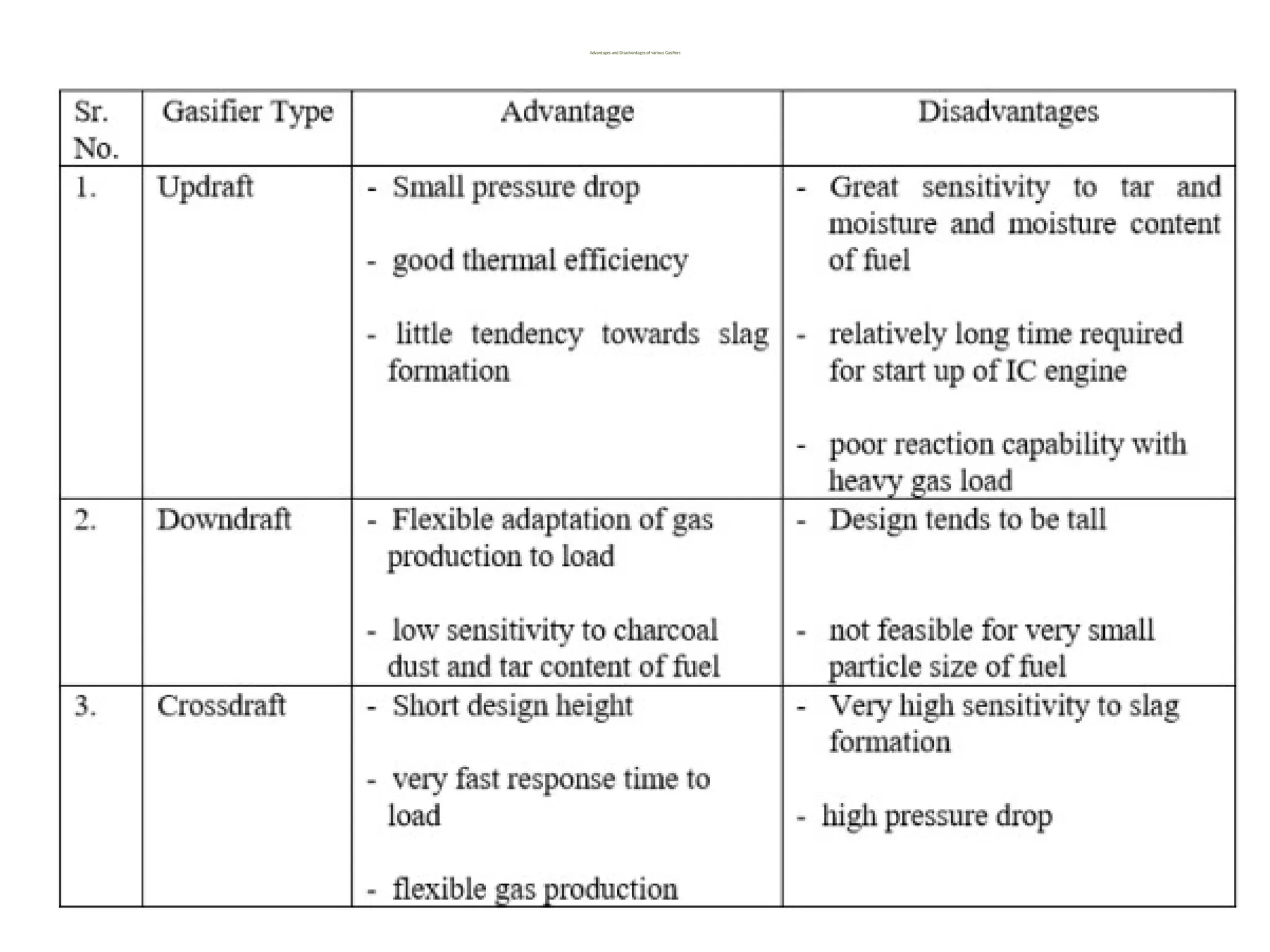
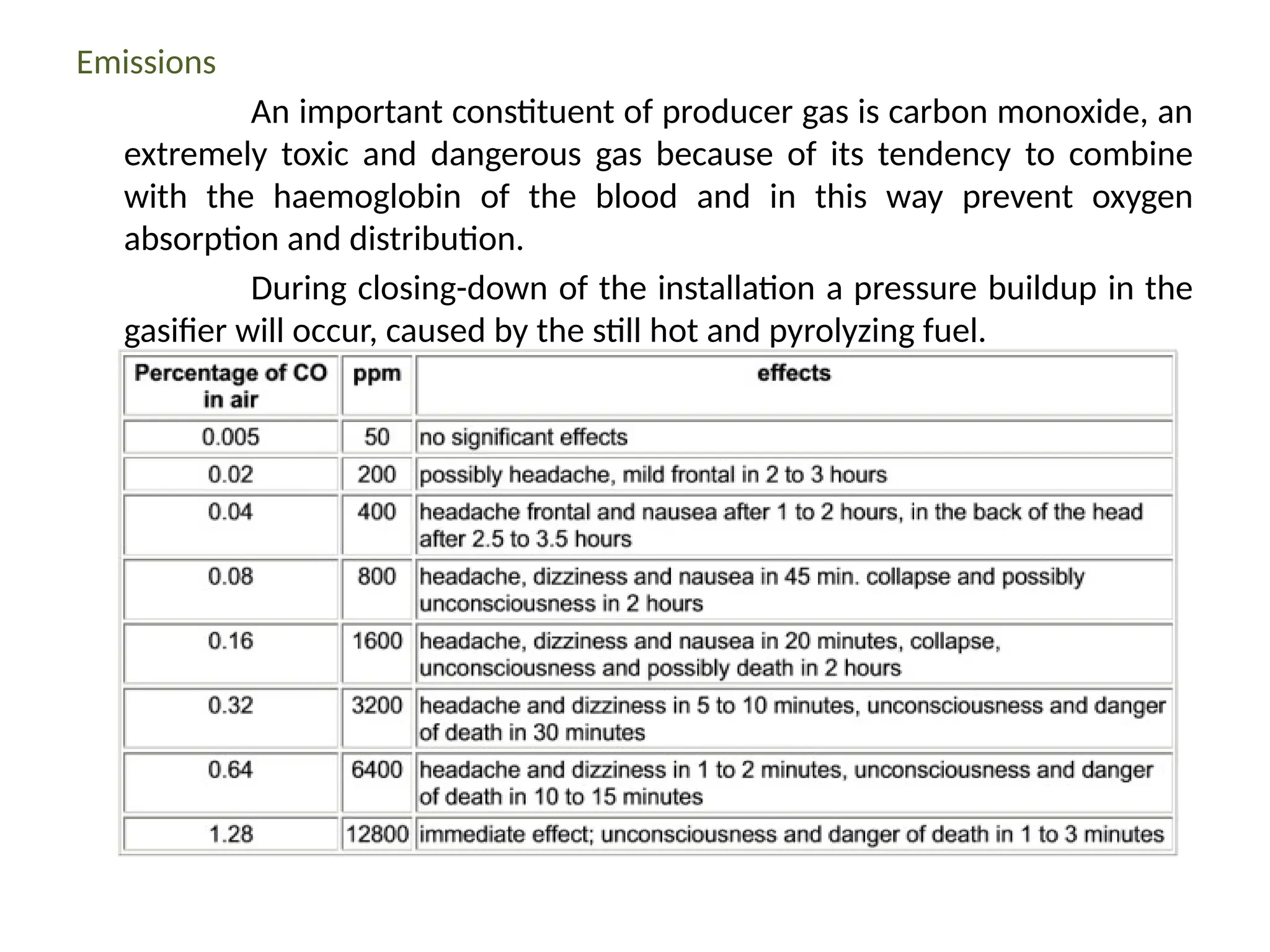
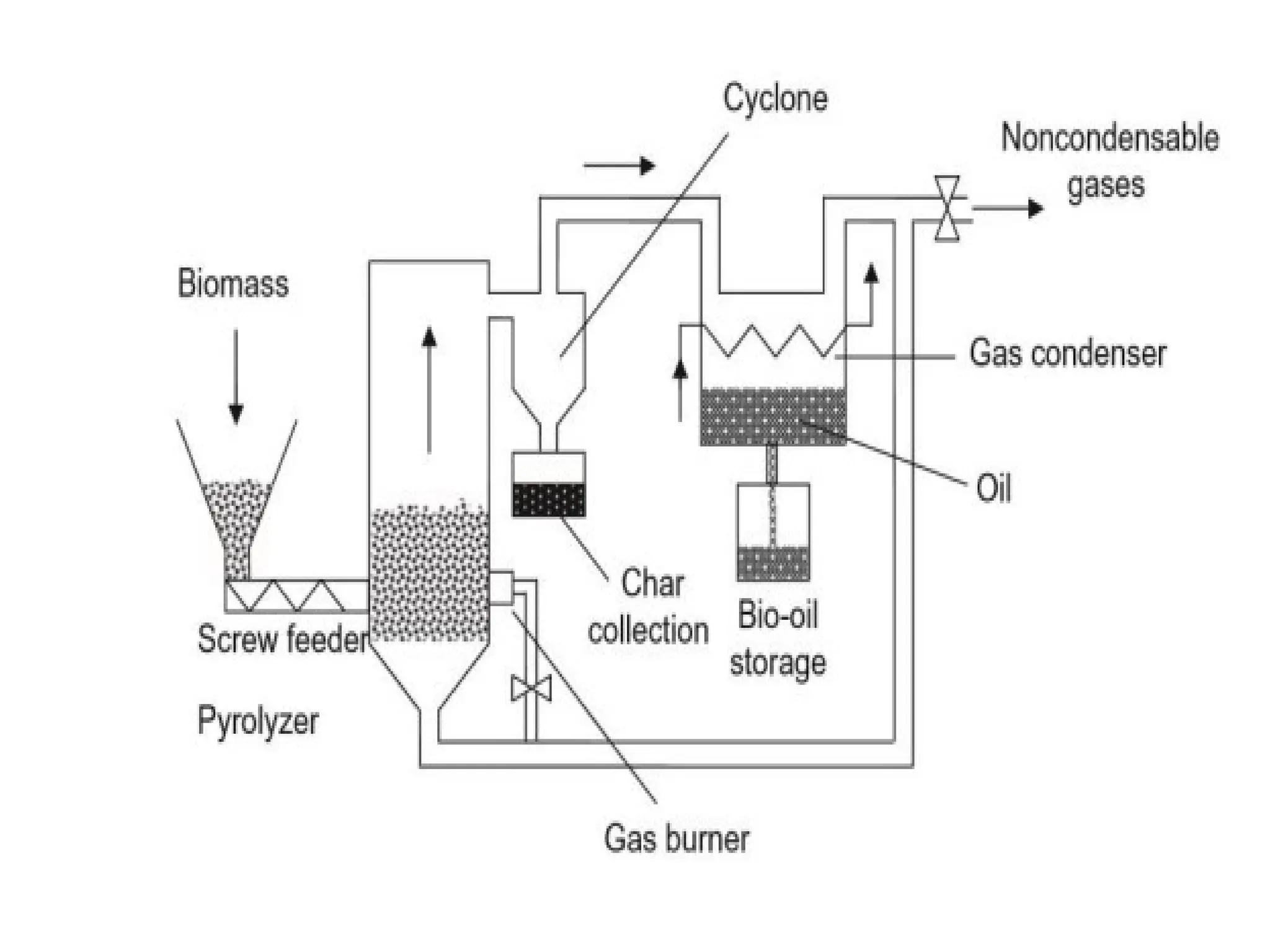
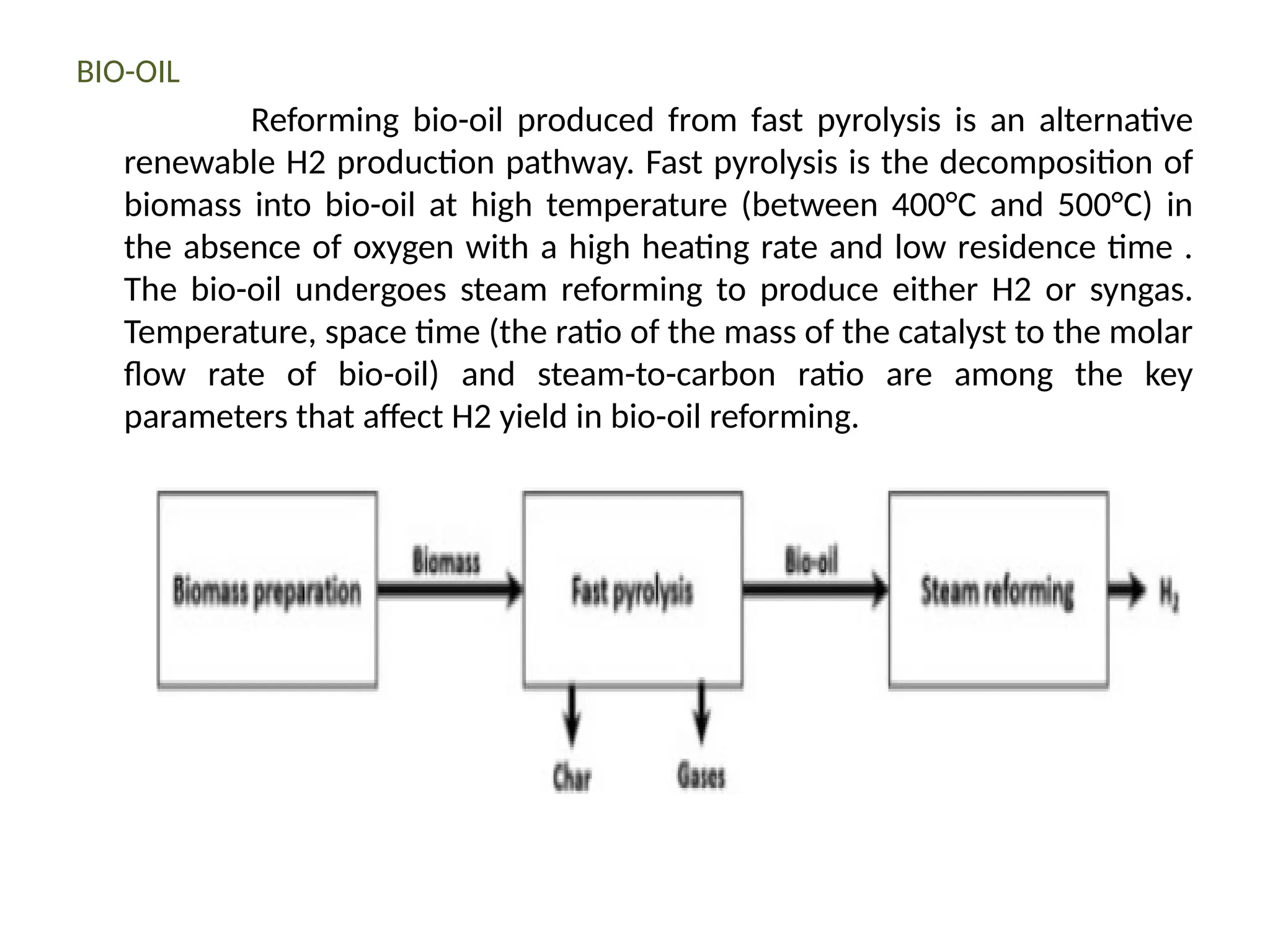
The document provides an overview of biomass gasification and its processes, highlighting the production of combustible gases like carbon monoxide and hydrogen from incomplete combustion. It discusses various types of gasifiers, their advantages, and limitations, as well as other biomass conversion methods such as pyrolysis and torrefaction. The content emphasizes the significance of utilizing biomass for energy production while addressing challenges like storage, transport, and gas cleaning.