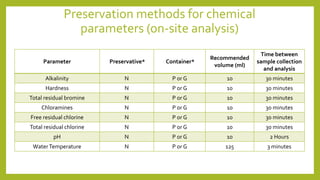
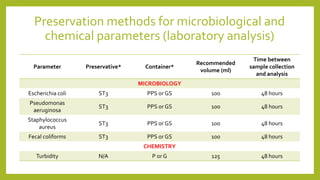
This document discusses the preservation of water samples for environmental engineering. It defines preservation as maintaining the original properties of water samples and explains that preservation is necessary to prevent changes in analyte concentrations between sample collection and laboratory analysis. It then describes common preservation methods like chemical addition, pH control, refrigeration, and freezing. Specific details are provided on preservation containers, techniques for various chemical and microbiological parameters, and required sampling volumes and holding times. The document serves to guide students on proper sample collection, preservation, and handling for environmental analysis.