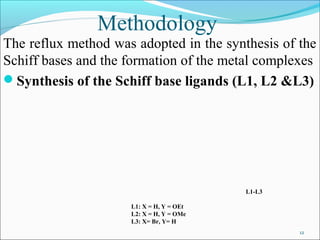
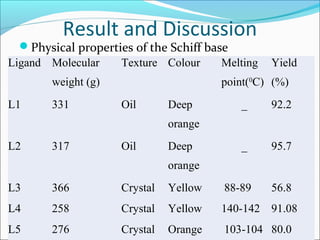
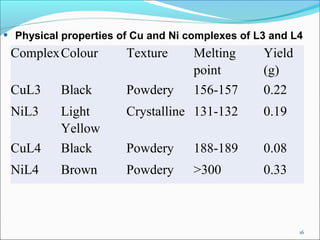
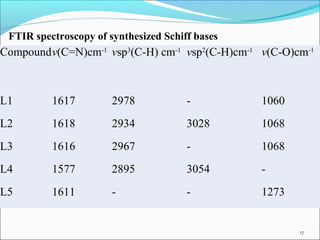
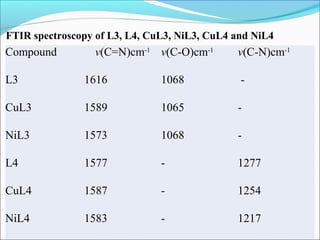
This document summarizes the synthesis and characterization of new Schiff base ligands and their metal complexes. It describes the synthesis of five Schiff base ligands derived from substituted benzaldehydes and anilines using the reflux method. Copper and nickel complexes were formed from two of the ligands. The ligands and complexes were characterized using infrared spectroscopy, which showed shifts in the C=N and C-O peaks upon complexation. The research aims to synthesize new Schiff bases and their Cu2+ and Ni2+ complexes and characterize them using IR spectroscopy to determine the coordination sites.