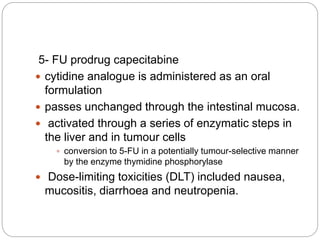
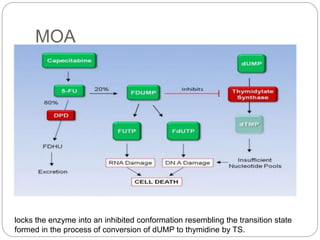
Alkylating agents and antimetabolites are two classes of chemotherapy drugs. Alkylating agents work by binding to DNA and RNA, causing crosslinking or breaks that prevent replication. The main types are nitrogen mustards, alkyl sulphonates, nitrosoureas, and thiazines. Antimetabolites mimic normal metabolites and inhibit DNA or RNA synthesis by becoming incorporated. Major types are folate antagonists like methotrexate, pyrimidine analogs like 5-fluorouracil, and purine analogs like mercaptopurine. Both classes cause bone marrow suppression and gastrointestinal toxicity, and resistance can develop through drug inactivation or changes to drug targets.