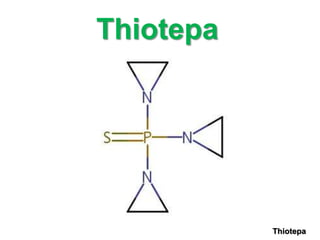
Alkylating agents are a class of chemotherapy drugs that work by alkylating DNA. Key points about alkylating agents from the document include:
1) Common alkylating agents discussed include cyclophosphamide, ifosfamide, melphalan, chlorambucil, thiotepa, busulfan, and nitrosoureas.
2) They work by adding alkyl groups to DNA, forming covalent bonds that lead to cross-linking of DNA strands and cell death.
3) Toxicities include myelosuppression, nausea/vomiting, alopecia, and secondary cancers, among others. Special precautions are often required due to toxicities.