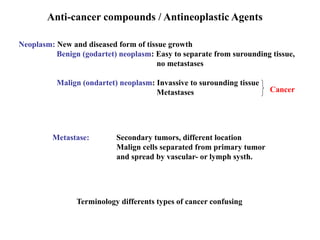
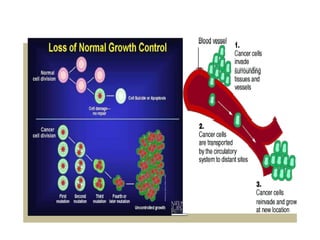
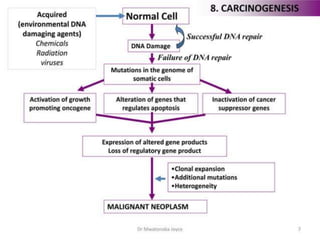
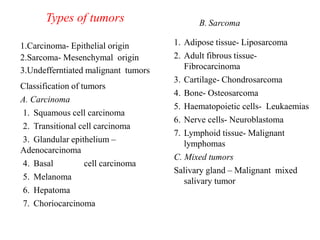
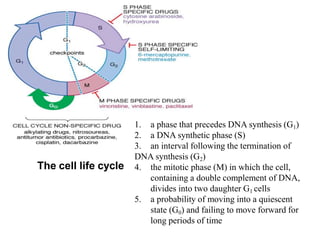
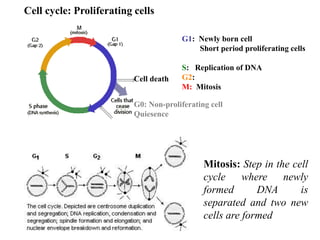
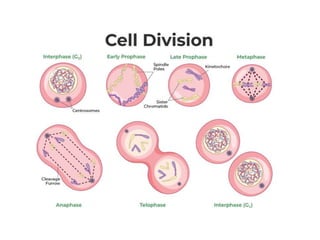
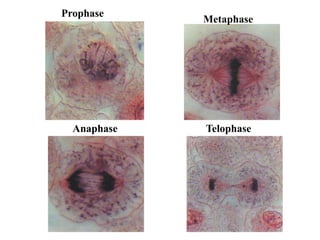
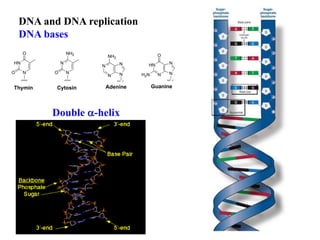
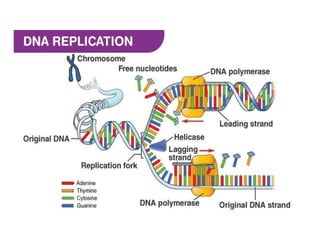
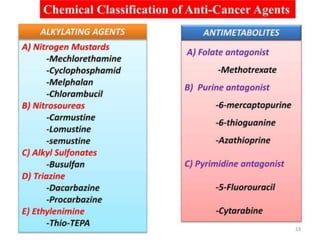
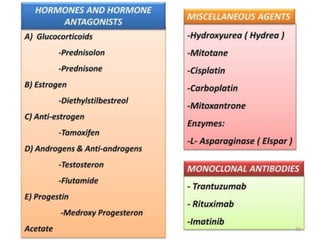
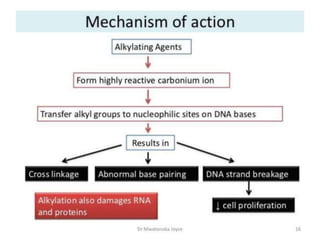
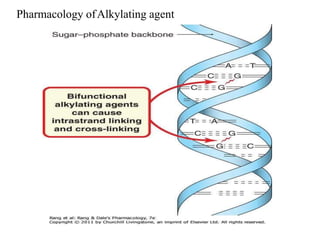
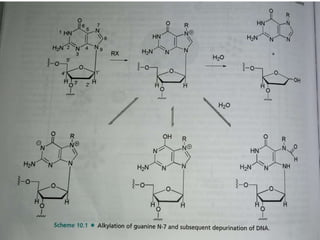
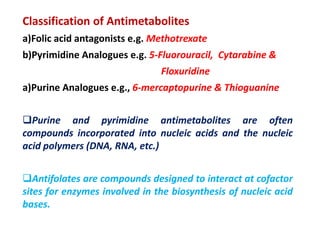
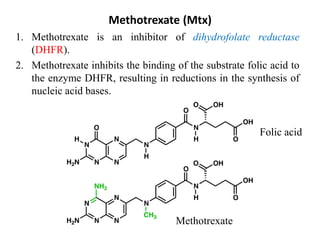
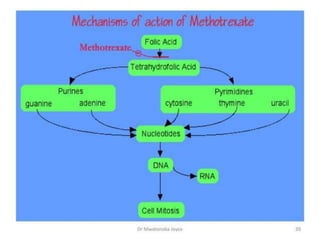
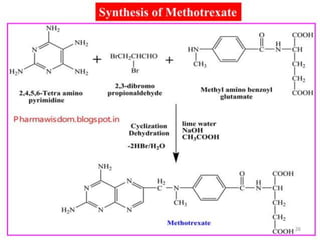
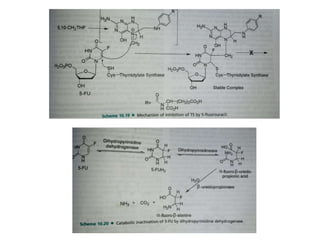
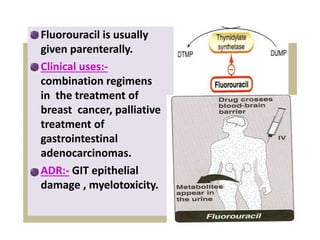
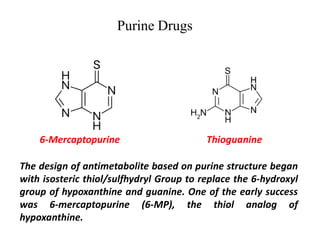
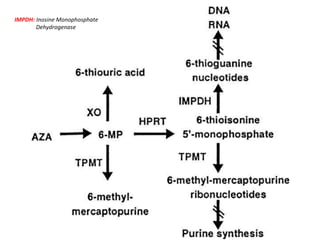
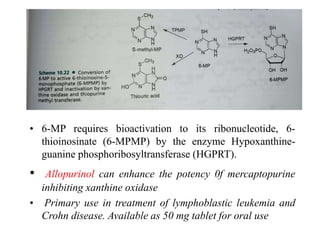
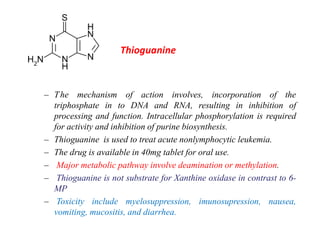
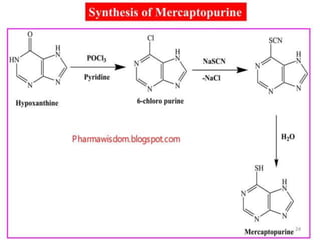
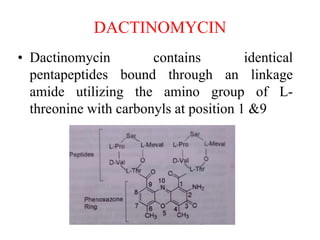
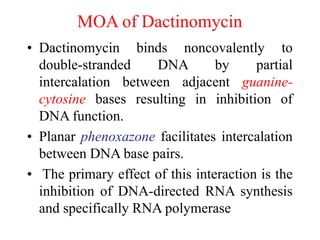
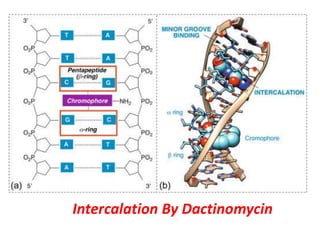
This document provides information on various types of antineoplastic (anti-cancer) agents, including their mechanisms of action and pharmacological properties. It discusses alkylating agents such as cyclophosphamide and cisplatin, which damage DNA and prevent cell replication. It also covers antimetabolites that interfere with nucleic acid synthesis, including methotrexate which inhibits dihydrofolate reductase, and 5-fluorouracil which inhibits thymidylate synthetase. The document provides details on the classification, mechanisms, uses and side effects of different classes of antineoplastic agents.



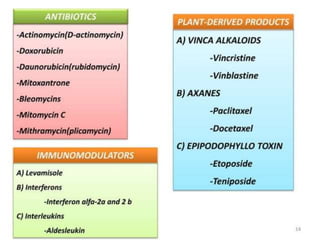







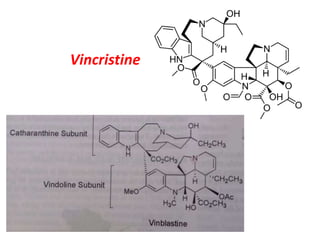
![Vincristine & Vinblastine
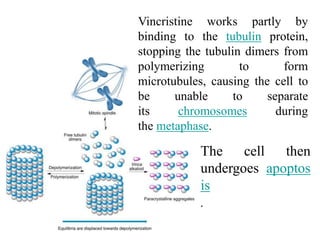
• Vincristine is delivered
via intravenous infusion for use in various
types of chemotherapy regimens.[3] Its main
uses are in non-Hodgkin's lymphoma
• The main side effects of vincristine
are chemotherapy-induced peripheral
neuropathy, hyponatremia, constipation,
and hair loss](https://image.slidesharecdn.com/anticancercompounds-230807033409-9bfdff24/85/Anticancer-compounds-ppt-73-320.jpg)