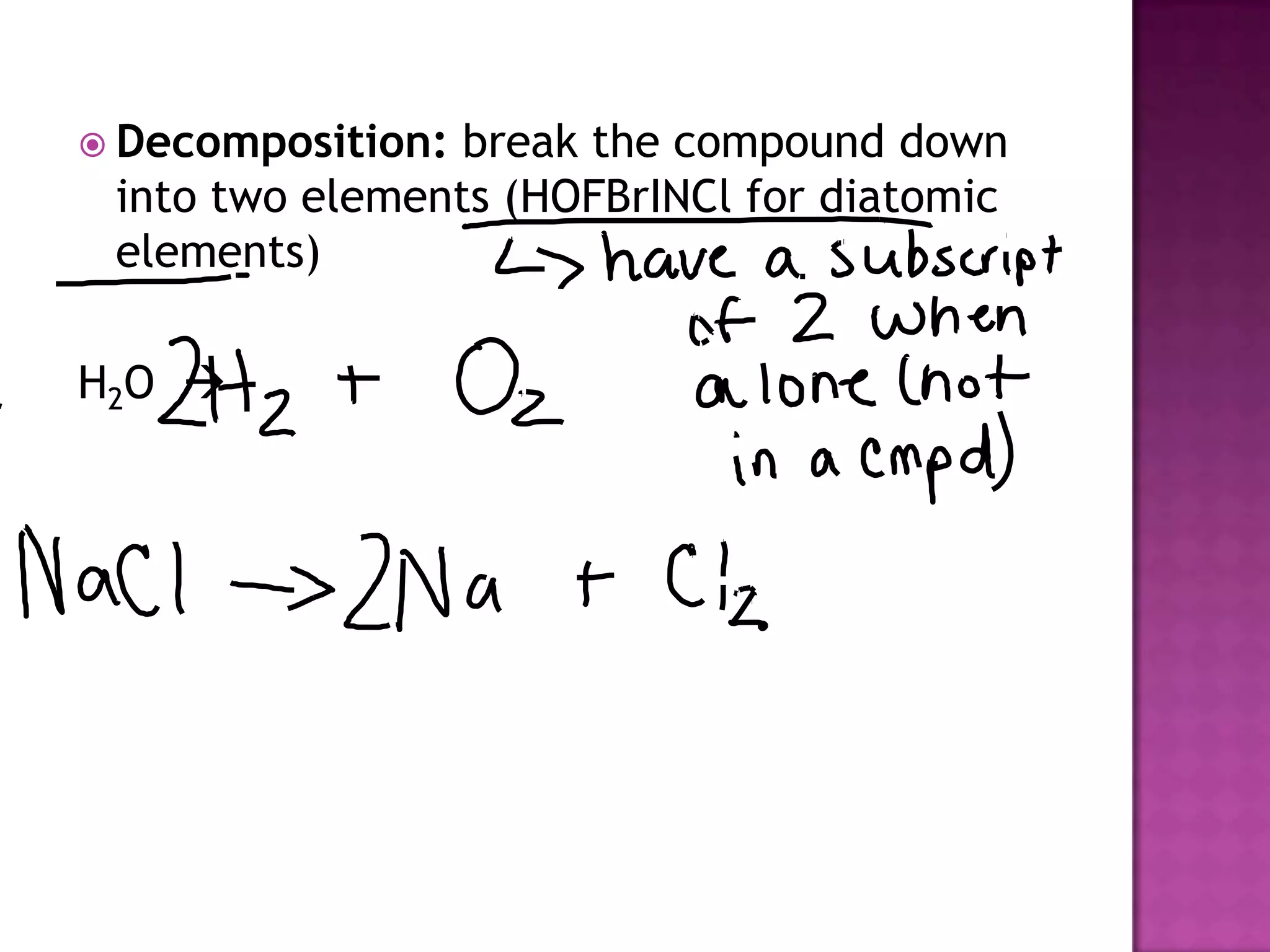
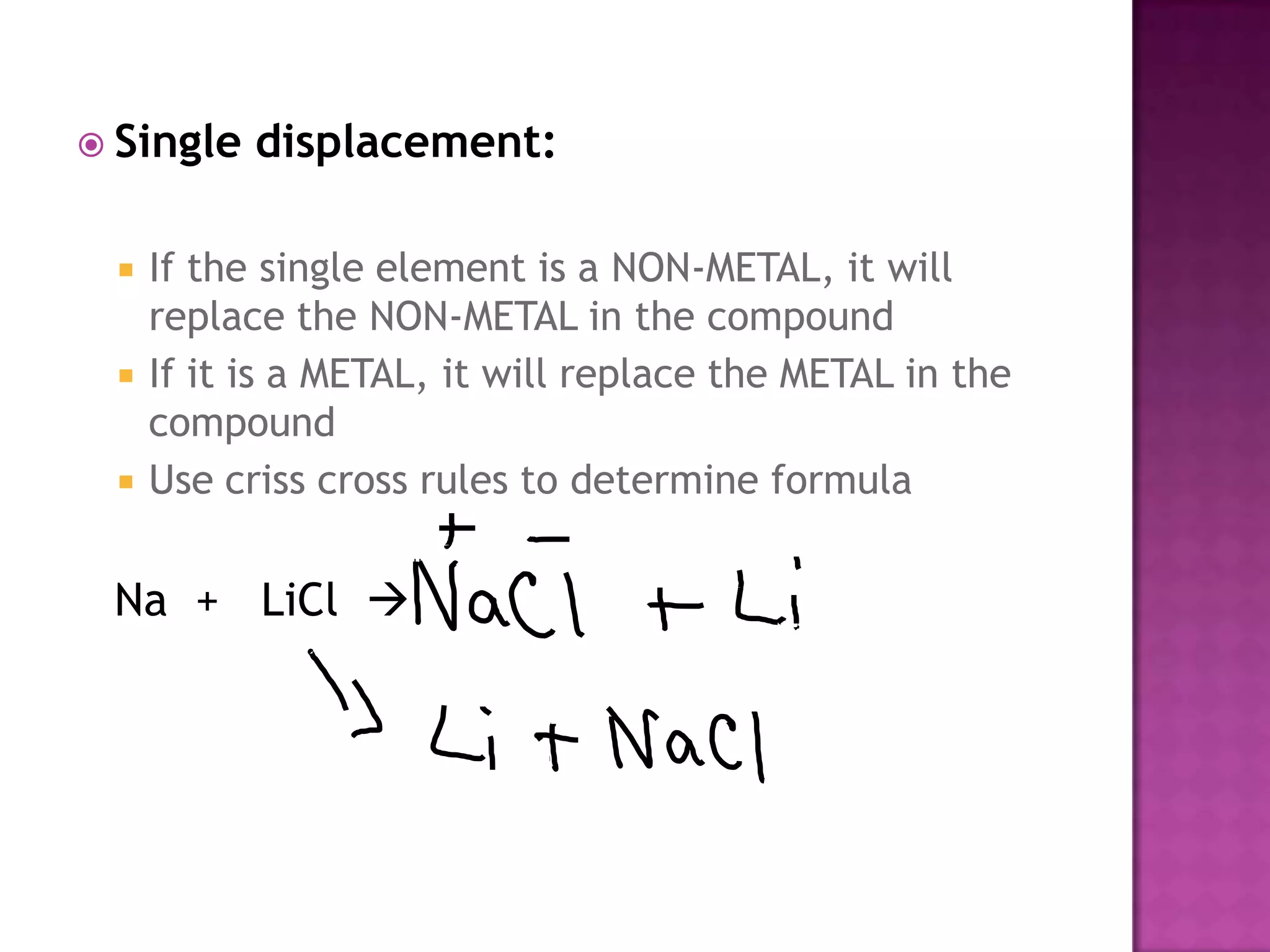
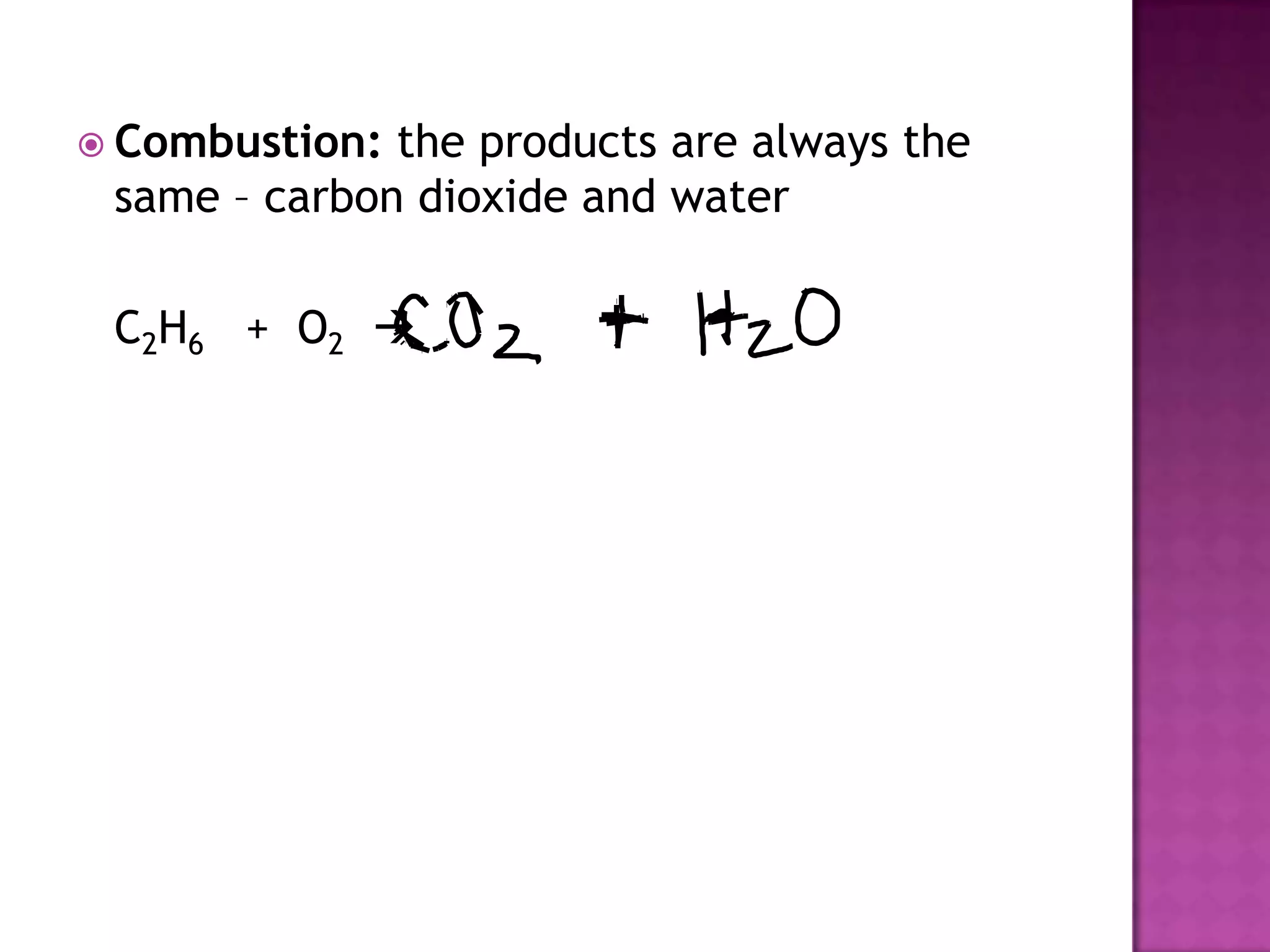
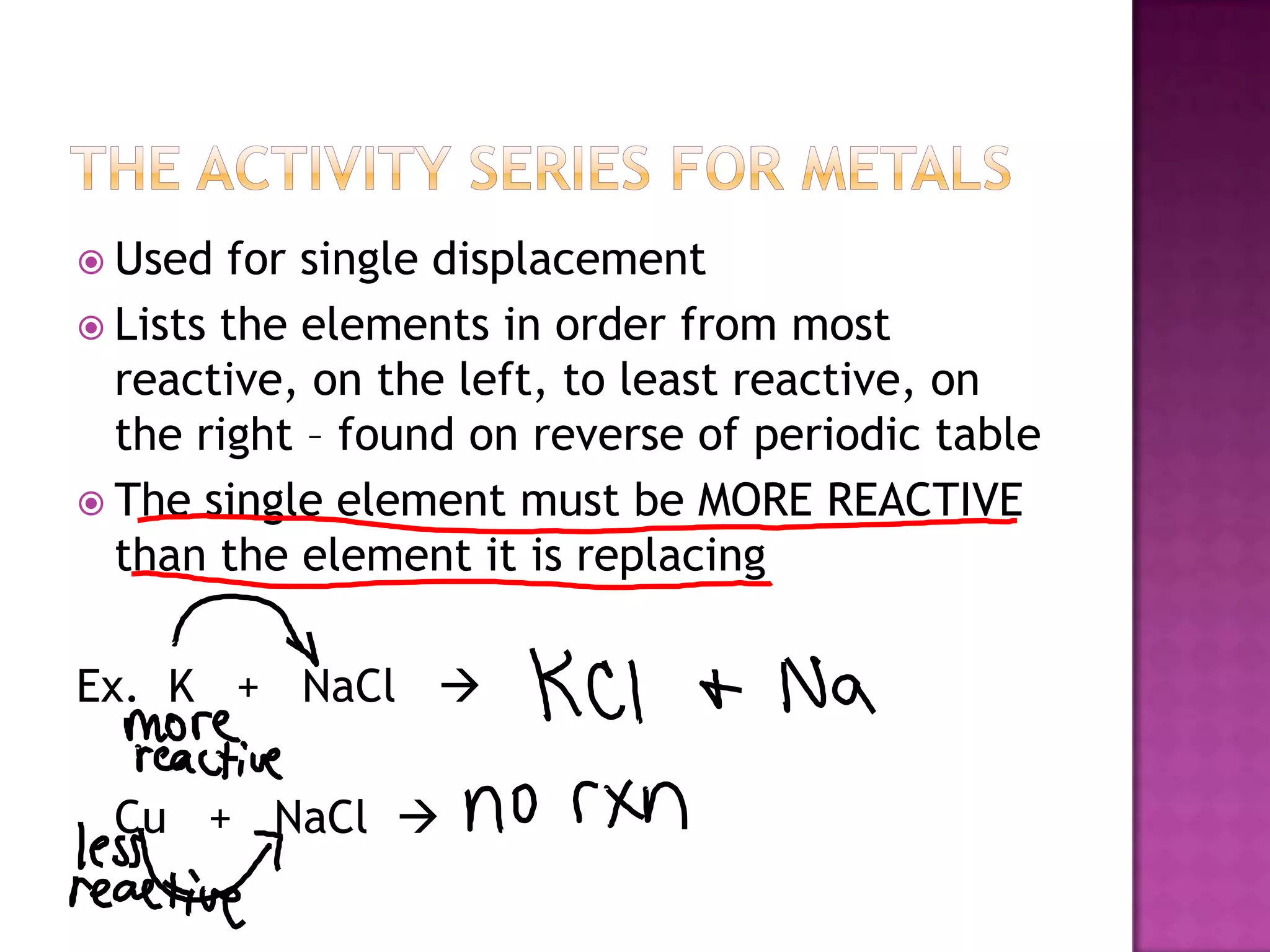
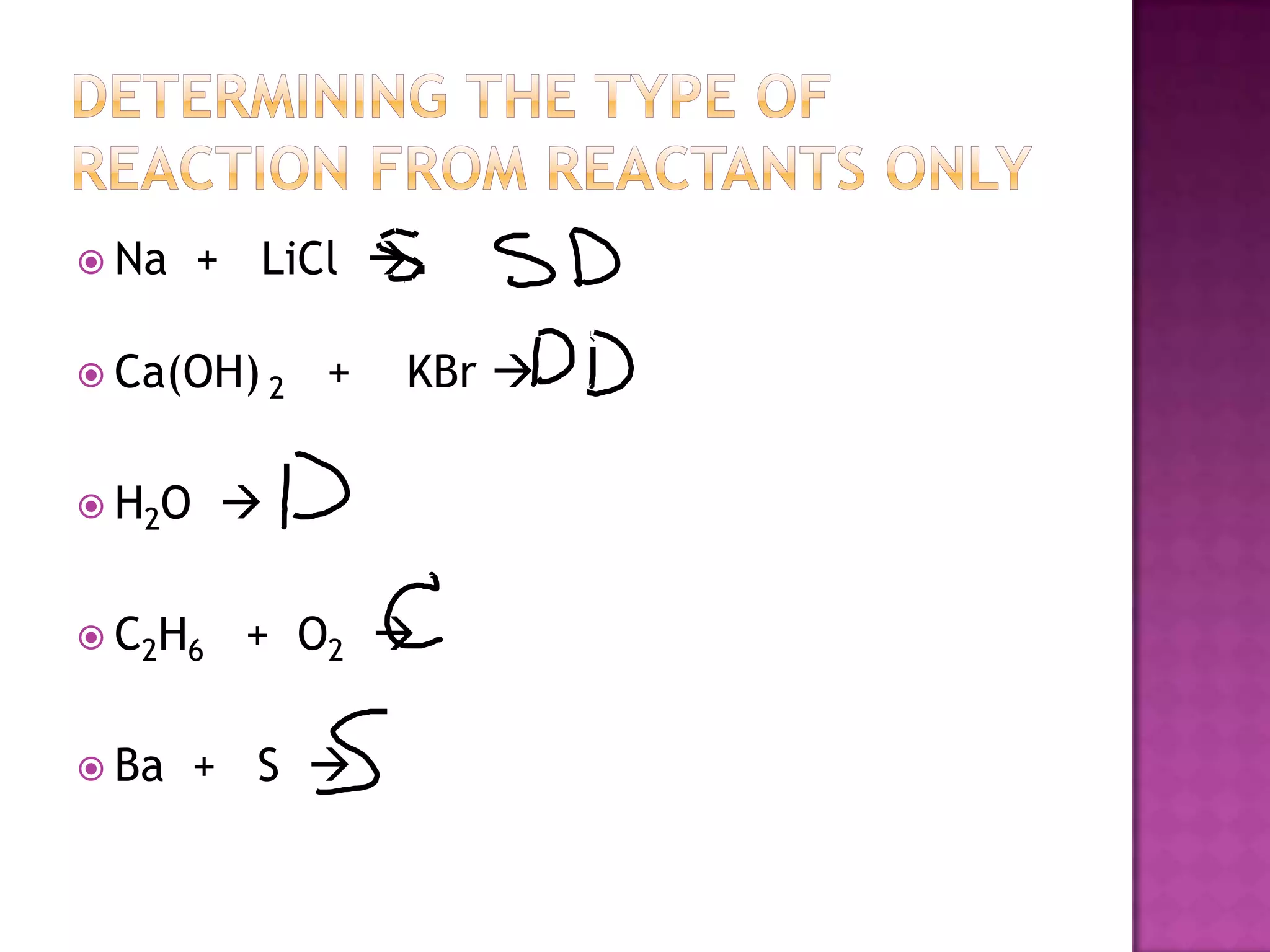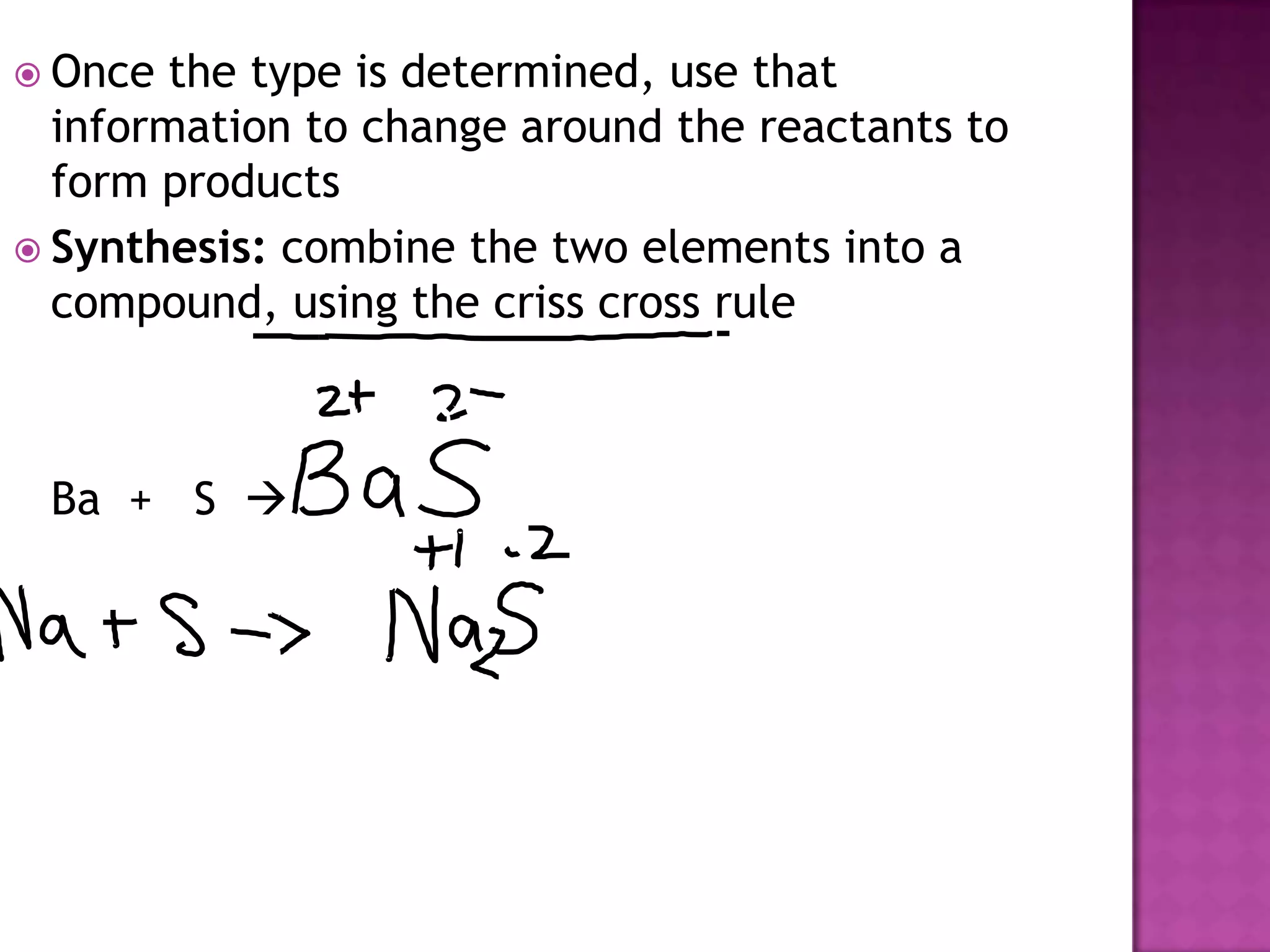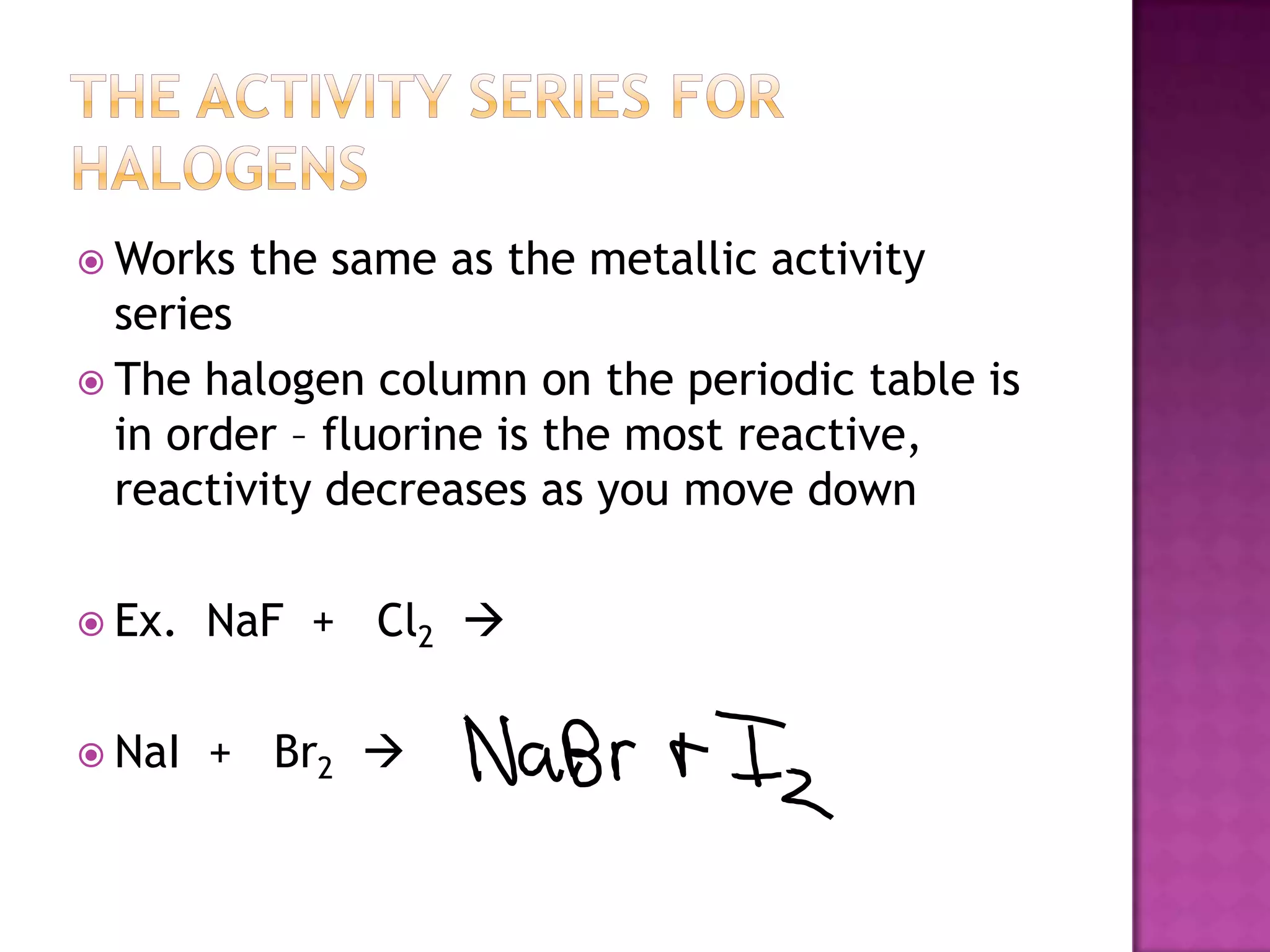The document discusses different types of chemical reactions including synthesis, decomposition, single displacement, double displacement, and combustion. It provides examples of each type of reaction using common reactants like Na, LiCl, Ca(OH)2, KBr, H2O, C2H6, O2, Ba, and S. Rules for determining products using the activity series on the periodic table and solubility rules are also covered.