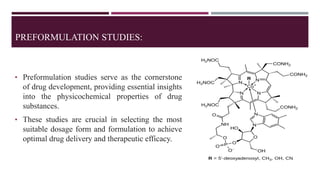
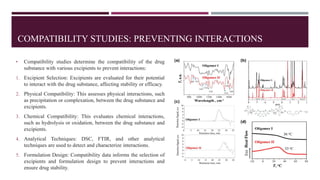
Preformulation studies are essential in drug development, providing critical insights into the physicochemical and biological properties of drug substances. These studies optimize formulation design, predict drug performance, reduce development risks, ensure regulatory compliance, and ultimately enhance product quality. Key areas include physicochemical characterization, solubility studies, stability assessments, and compatibility evaluations, all of which guide the formulation and development of effective and safe drug products.